Steviol glycoside sweetening agent with good mouth feel and capable of reducing blood sugar
A steviol glycoside and hypoglycemic technology, which is applied in the direction of food ingredients as mouthfeel improvers, food ingredients as taste improvers, functions of food ingredients, etc., can solve the problems of application limitations, sweetener product lingering aftertaste, heavy aftertaste, etc. , to achieve the effects of improving liver function, strengthening medical and health care effects, and reducing aftertaste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
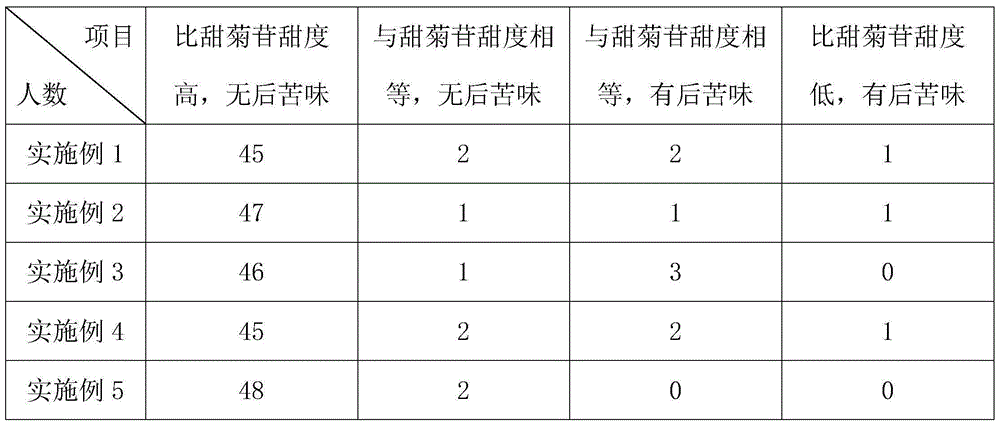
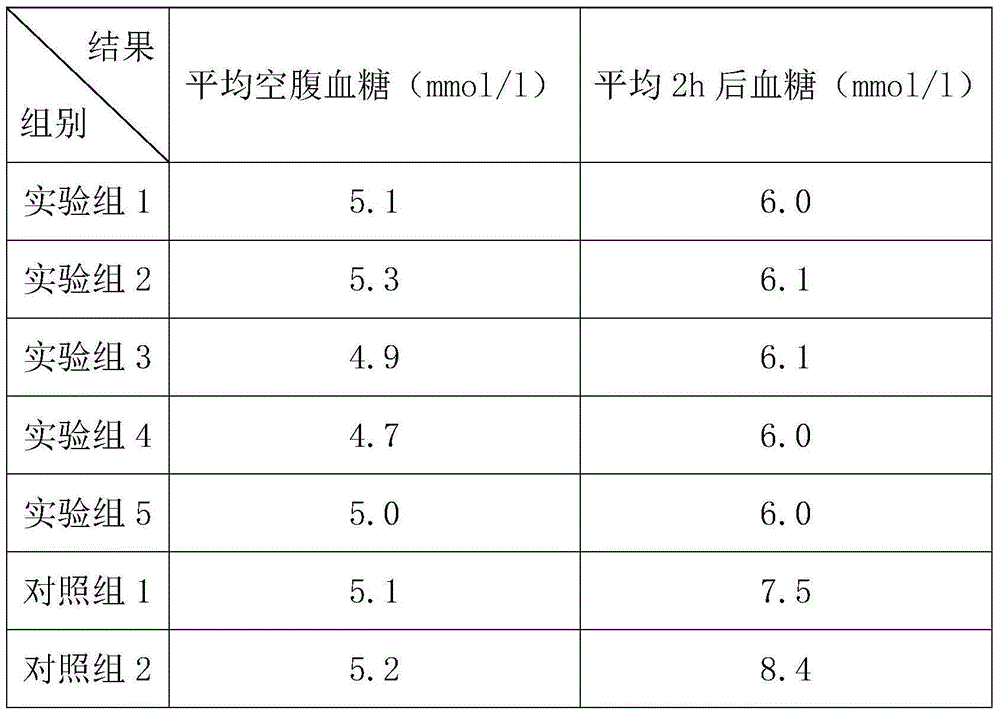
Embodiment 1
[0026] A good-tasting and hypoglycemic steviol glycoside sweetener. Its raw materials include 5 parts by weight of soybean oligosaccharides, 12 parts of fructooligosaccharides, 5 parts of erythritol, 15 parts of xylitol, and 5 parts of maltitol. 15 parts of thaumatin, 10 parts of modified stevioside, 50 parts of rebaudioside A, 2 parts of Salvia miltiorrhiza extract, 2 parts of astragalus polysaccharide, 1 part of Panax notoginseng extract, 4 parts of malt polysaccharide, 1 part of mulberry polysaccharide, 6 parts of Glycyrrhizin, 1 part of Platycodon grandiflorum polysaccharide;
[0027] Among them, the preparation method of modified stevioside is: dissolving sucrose and stevioside in a sodium dihydrogen phosphate-disodium hydrogen phosphate buffer with a concentration of 0.05 mol / l, wherein the pH of the buffer is 8 and adding sucrose invertase Mix well to obtain solution A, where the molar volume (mol / l) ratio of stevioside to buffer is 0.0015:1, the molar volume (mol / l) ratio...
Embodiment 2
[0031] A good-tasting and hypoglycemic steviol glycoside sweetener, whose raw materials by weight include: 10 parts of soybean oligosaccharides, 8 parts of fructooligosaccharides, 7 parts of erythritol, 5 parts of xylitol, and 10 parts of maltitol. 10 parts of thaumatin, 30 parts of modified stevioside, 30 parts of rebaudioside A, 3 parts of salvia miltiorrhiza extract, 1 part of astragalus polysaccharide, 3 parts of Panax notoginseng extract, 2 parts of malt polysaccharide, 2 parts of mulberry polysaccharide, 4 parts of Glycyrrhizin and 3 parts of Platycodon grandiflorum;
[0032] Among them, the preparation method of modified stevioside is: dissolving sucrose and stevioside in a sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution with a concentration of 0.07 mol / l, wherein the pH of the buffer solution is 6 and adding sucrose invertase Mix well to obtain solution A, where the molar volume (mol / l) ratio of stevioside to buffer is 0.002:1, the molar volume (mo...
Embodiment 3
[0036] A good-tasting and hypoglycemic steviol glycoside sweetener, whose raw materials by weight include: 9 parts of soybean oligosaccharides, 9 parts of fructooligosaccharides, 6.5 parts of erythritol, 8 parts of xylitol, and 8 parts of maltitol. Somatin 12 parts, modified stevioside 25 parts, rebaudioside A 35 parts, salvia miltiorrhiza extract 2.8 parts, astragalus polysaccharide 1.3 parts, panax notoginseng extract 2.5 parts, malt polysaccharide 2.5 parts, mulberry polysaccharide 1.8 parts, 4.5 parts of Glycyrrhizin, 2.5 parts of Platycodon grandiflorum polysaccharide;
[0037] Wherein, the preparation method of modified stevioside is: dissolving sucrose and stevioside in a sodium dihydrogen phosphate-disodium hydrogen phosphate buffer with a concentration of 0.065 mol / l, wherein the pH of the buffer is 6.5, adding sucrose invertase Mix well to obtain solution A, where the molar volume (mol / l) ratio of stevioside to buffer is 0.0019:1, the molar volume (mol / l) ratio of sucro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com