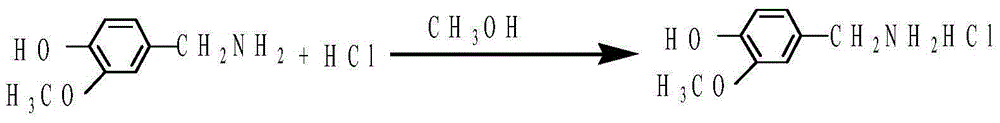
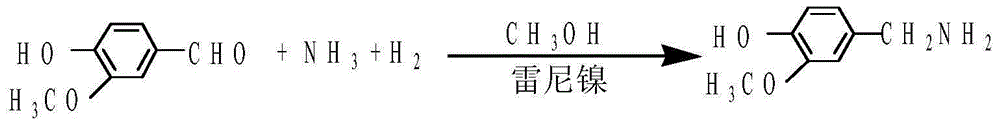Preparation method of 3-methoxy-4-hydroxybenzylamine hydrochloride
A technology of hydroxybenzylamine hydrochloride and hydroxybenzaldehyde, which is applied in the field of chemical reagent synthesis technology, can solve the problems of low yield and high cost of the synthesis process, and achieve the effects of high product precision, reduced raw material costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The present embodiment provides a kind of preparation method of 3-methoxyl-4-hydroxybenzylamine hydrochloride specifically comprising the following steps:
[0022] 1) Dissolve 75g (0.49mol) of 3-methoxy-4-hydroxybenzaldehyde in 200g of anhydrous methanol in advance for later use.
[0023] 2) Pump the 3-methoxy-4-hydroxybenzaldehyde solution ready for use in step 1) into a 1L autoclave, start stirring, add 8g of Raney nickel catalyst, add 220g of anhydrous methanol, and close For all valves in the autoclave, first use vacuum to take away most of the air, and then introduce nitrogen until the pressure in the autoclave reaches 3kg / c㎡, and the pressure does not change. After confirming that the autoclave is well sealed, the nitrogen is discharged, and the operation is repeated 2 to 3 times. , replace all the air in the autoclave with nitrogen.
[0024] 3) Turn on the cooling water in the jacket of the autoclave, control the temperature in the autoclave to below 35°C, add 1...
Embodiment 2
[0033] The present embodiment provides a kind of preparation method of 3-methoxyl-4-hydroxybenzylamine hydrochloride specifically comprising the following steps:
[0034] 1) Dissolve 75g (0.49mol) of 3-methoxy-4-hydroxybenzaldehyde in 150g of anhydrous methanol in advance for later use.
[0035] 2) Pump the 3-methoxyl-4-hydroxybenzaldehyde solution ready for use in step 1) into a 1L autoclave, start stirring, add 6.25g of Raney nickel catalyst, and then add 220g of anhydrous methanol , close all the valves of the autoclave, first take away most of the air with vacuum, and then introduce nitrogen until the pressure in the autoclave reaches 3kg / c㎡, and there is no change in the pressure, confirm that the seal of the autoclave is intact, and then discharge the nitrogen, repeat the operation 2~ Three times, all the air in the autoclave was replaced with nitrogen.
[0036] 3) Turn on the cooling water in the jacket of the autoclave, control the temperature in the autoclave to belo...
Embodiment 3
[0042] 1) Dissolve 75g (0.49mol) of 3-methoxy-4-hydroxybenzaldehyde in 300g of anhydrous methanol in advance for later use.
[0043] 2) Pump the 3-methoxyl-4-hydroxybenzaldehyde solution ready for use in step 1) into a 1L autoclave, start stirring, add 9.4g of Raney nickel catalyst, and then add 220g of anhydrous methanol , close all the valves of the autoclave, first take away most of the air with vacuum, and then introduce nitrogen until the pressure in the autoclave reaches 3kg / c㎡, and there is no change in the pressure, confirm that the seal of the autoclave is intact, and then discharge the nitrogen, repeat the operation 2~ Three times, all the air in the autoclave was replaced with nitrogen.
[0044] 3) Turn on the cooling water in the jacket of the autoclave, control the temperature in the autoclave to below 35°C, add 225g (13.2mol) of liquid ammonia to the autoclave, and the rate of adding liquid ammonia is to control the temperature in the autoclave to below 50°C.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com