Reactive flame retardants adopting bridged cyclophosphazene and preparation methods of reactive flame retardants
A technology based on cyclotriphosphazene and chlorinated cyclotriphosphazene is applied in the field of bridged cyclophosphazene reactive flame retardant and its preparation, and can solve the problem of poor thermal stability, limited environmental conditions, and lowering the initial thermal decomposition temperature of the matrix, etc. problem, to achieve the effect of good thermal stability, large steric hindrance and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
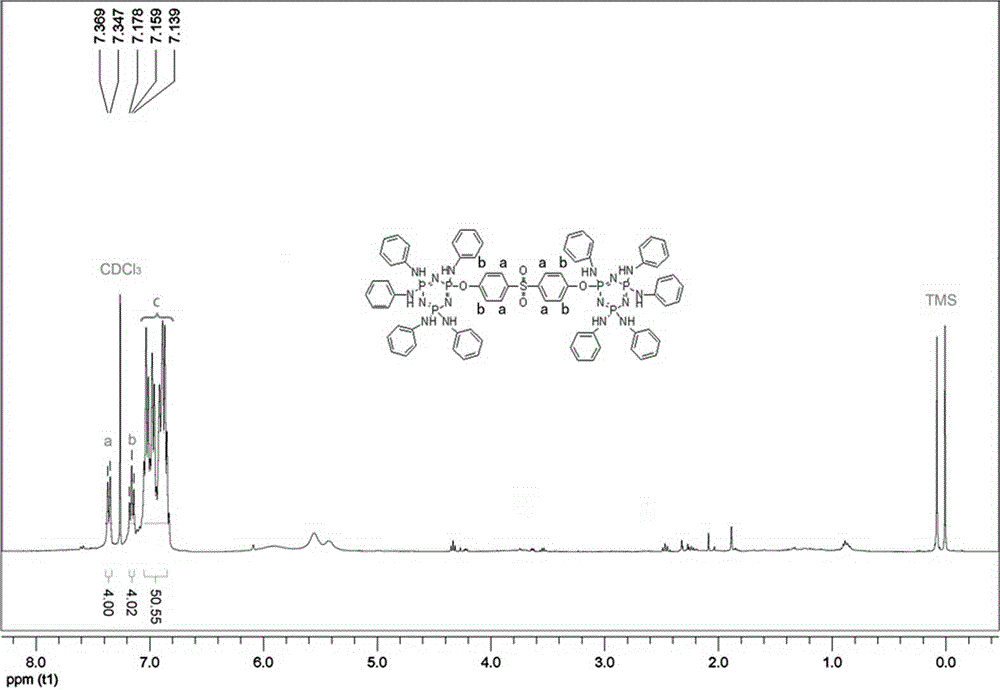
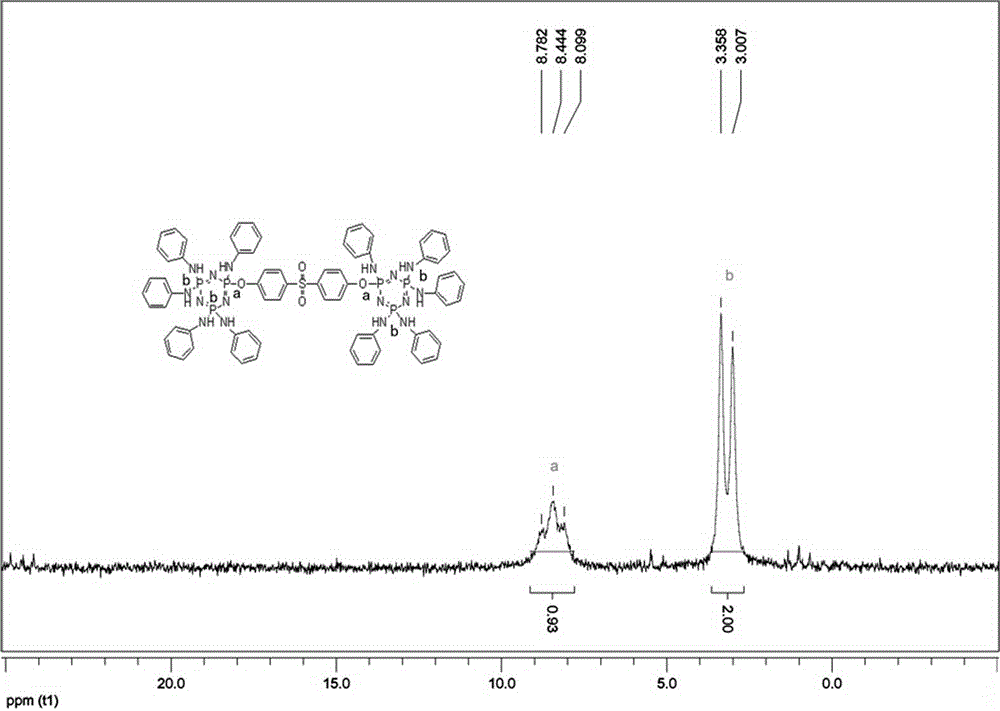
[0025] In a 250mL round-bottomed flask, nitrogen gas was introduced, and 56.0g (0.6mol) of aniline, 16.0g (0.12mol) of potassium carbonate, and 10.0g (0.012mol) of bisphenol S bridged chlorinated cyclotriphosphazene (BCP-BPS) were added. ). Stir at room temperature and react for 2h; heat up to 80°C, react for 2h; heat up to reflux, react for 12h. After filtration, the filtrate was distilled under reduced pressure to remove aniline to obtain a yellow solid. Wash 3 times with deionized water and dry to remove residual moisture. Recrystallized three times using diethyl ether / petroleum ether to obtain 15.3 g of a light yellow solid with a yield of 93.0%.
Embodiment 2
[0027] In a 250mL round-bottomed flask, nitrogen gas was introduced, and 56.0g (0.6mol) of aniline, 12.7g (0.12mol) of sodium carbonate, and 10.0g (0.012mol) of bisphenol S bridged chlorinated cyclotriphosphazene (BCP-BPS) were added. ). The reaction was stirred at room temperature for 3 hours; the temperature was raised to 80° C., and the reaction was made for 4 hours; the temperature was raised to reflux, and the reaction was made for 14 hours. After filtration, the filtrate was distilled under reduced pressure to remove aniline to obtain a yellow solid. Wash 5 times with deionized water and dry to remove residual moisture. Recrystallized three times using diethyl ether / petroleum ether to obtain 14.6 g of light yellow solid with a yield of 89.0%.
Embodiment 3
[0029] Into a 500mL round-bottomed flask, nitrogen was introduced, and 279.0g (3.0mol) of aniline, 80.0g (0.6mol) of potassium carbonate, and 50.0g (0.06mol) of bisphenol S bridged chlorinated cyclotriphosphazene (BCP-BPS) were added. ). Stir the reaction at room temperature for 4 hours; raise the temperature to 80° C., and react for 3 hours; raise the temperature to reflux, and react for 24 hours. After filtration, the filtrate was distilled under reduced pressure to remove aniline to obtain a yellow solid. Wash 4 times with deionized water and dry to remove residual moisture. Recrystallized three times using diethyl ether / petroleum ether to obtain 75.0 g of light yellow solid with a yield of 91.0%.
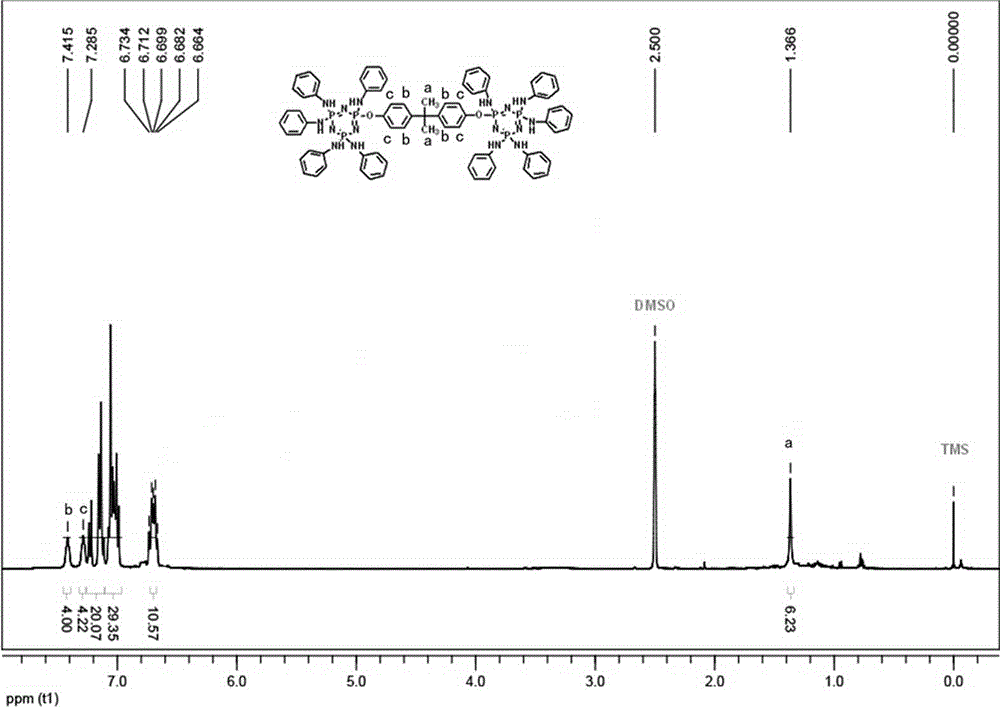
[0030] (2) Bisphenol A bridged anilino cyclotriphosphazene compound
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com