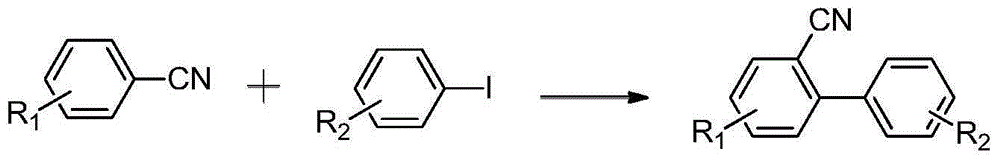Synthesis method of dicyano substituted biphenyl compounds
A synthesis method and compound technology are used in the synthesis of dicyano-substituted biphenyl compounds, organic synthesis, and the synthesis of biphenyl compounds, and achieve the effect of good application prospects and industrial production potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
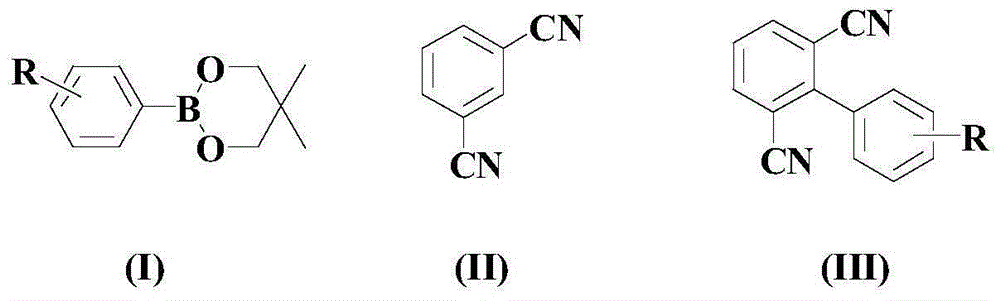
[0033] Under a nitrogen atmosphere and room temperature, add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, and 3 mmol of catalyst to an appropriate amount of organic solvent (an equal-volume mixture of 1,4-dioxane and PEG-200) (for 1mmolNiCl 2 (PPh 3 ) 2 Mixture with 2mmol CuOTf), 10mmol ligand L1 and 10mmol activator NFSI, warming up to 80°C and stirring at this temperature for 8 hours;
[0034] After the reaction was completed, the reaction mixture was naturally cooled to room temperature, and a mixed solution of ethyl acetate and deionized water with a volume ratio of 1:1 was added thereto, fully oscillated, left to stand for stratification, and the organic phase was separated, and the water phase was used again. Ethyl acetate was extracted 2-3 times, all organic phases were combined, dried with anhydrous sodium sulfate, and distilled under reduced pressure, and the residue was subjected to 200-300 mesh silica gel col...
Embodiment 2
[0037]
[0038] In a nitrogen atmosphere and at room temperature, add 100 mmol of the above formula (I) compound, 170 mmol of the above formula (II) compound, and 5 mmol of catalyst to an appropriate amount of organic solvent (an equal volume mixture of 1,4-dioxane and PEG-200) (1.25mmolNiCl 2 (PPh 3 ) 2 Mixture with 3.75mmol CuOTf), 15mmol ligand L1 and 13mmol activator NFSI, warming up to 90°C and stirring at this temperature for 7 hours;
[0039]After the reaction was completed, the reaction mixture was naturally cooled to room temperature, and a mixed solution of ethyl acetate and deionized water with a volume ratio of 1:1 was added thereto, fully oscillated, left to stand for stratification, and the organic phase was separated, and the water phase was used again. Ethyl acetate was extracted 2-3 times, all organic phases were combined, dried with anhydrous sodium sulfate, and distilled under reduced pressure, and the residue was subjected to 200-300 mesh silica gel co...
Embodiment 3
[0042]
[0043] In a nitrogen atmosphere and at room temperature, add 100 mmol of the above formula (I) compound, 200 mmol of the above formula (II) compound, and 6 mmol of catalyst to an appropriate amount of organic solvent (an equal volume mixture of 1,4-dioxane and PEG-200) (1.8mmolNiCl 2 (PPh 3 ) 2 Mixture with 4.2mmol CuOTf), 20mmol ligand L1 and 16mmol activator NFSI, heated to 100°C and stirred at this temperature for 6 hours;
[0044] After the reaction was completed, the reaction mixture was naturally cooled to room temperature, and a mixed solution of ethyl acetate and deionized water with a volume ratio of 1:1 was added thereto, fully oscillated, left to stand for stratification, and the organic phase was separated, and the water phase was used again. Ethyl acetate was extracted 2-3 times, all organic phases were combined, dried with anhydrous sodium sulfate, and distilled under reduced pressure, and the residue was subjected to 200-300 mesh silica gel column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com