Cell culture fluid, application of cell culture fluid and method of inducting DPSCs to differentiate into neuron-like cells
A dental pulp stem cell and cell culture technology, which can be applied to non-embryonic pluripotent stem cells, animal cells, nervous system cells, etc., and can solve the problems of long cycle and low differentiation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
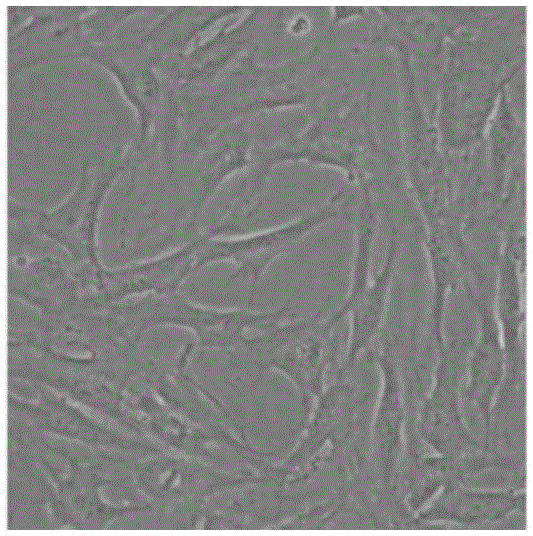
[0059] Using 3-5 passages of dental pulp stem cells, according to 2×10 3 piece / cm 2 Seeding Density Seeding was done in six-well plates. After cultivating 4h~6h cell adherence with DMEM / F12, after adding EGF pre-induction respectively, discard the complete medium, wash the cells twice with PBS, add the cell culture fluid (containing GDNF, Shh, EGF, cAMP) provided by the present invention DMEM / F12 serum-free medium), after induction of cultured cells. Observe the differentiation of dental pulp stem cells. The pre-induction conditions and cell culture medium components nuclear induction conditions used in each embodiment are shown in Table 1:
[0060] Table 1, Examples 1-5
[0061]
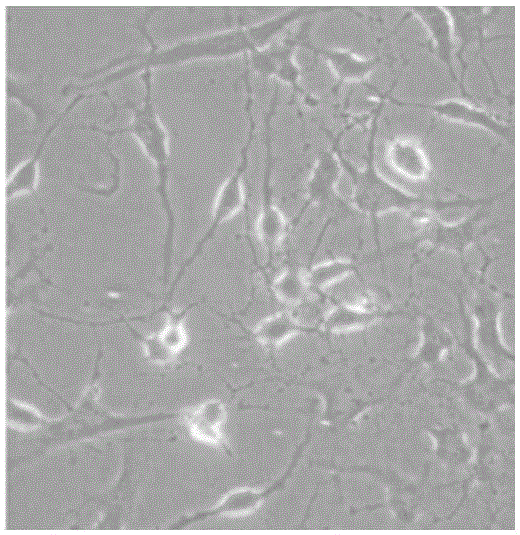
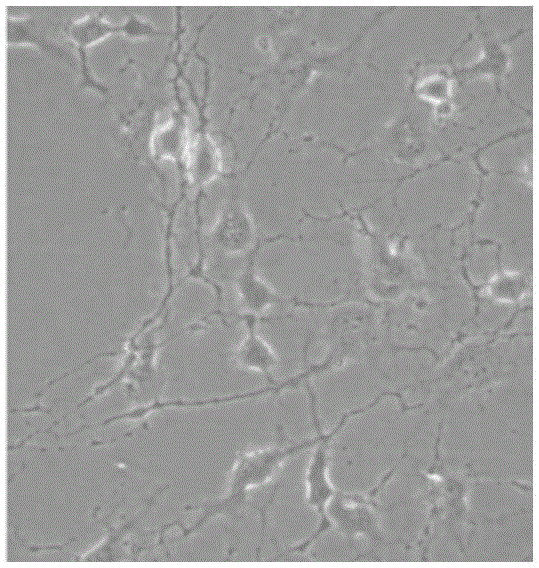
[0062] After the cells of each example were pre-induced and induced, the cell morphology was observed with an electron microscope. The results showed that the cell morphology began to change after 12 hours, and changed significantly after 24 hours of induction. The refraction of the cells beg...
Embodiment 6
[0069] Extract the total proteins with cells induced in Examples 1-5 and Comparative Examples 1-3, and perform Western blot to detect the changes in the expression of Nestin and MAP2 during the induction process. Specifically, the total proteins obtained by each extraction were polymerized with 12% SDS-PAGE. Acrylamide gel electrophoresis, transfer to PVDF membrane at 80V for 1h. Block with 5% BSA at room temperature for 1 h, add anti-Nestin, MAP2 primary antibody (1:200) or β-tubulin antibody (1:200), overnight at 4°C; wash the membrane, add HRP-labeled secondary antibody (1:2000), Incubate at room temperature for 60 min; ECL emits light. The result is as Figure 5 .
[0070] The results showed that the expressions of Nestin and MAP2 increased after the dental pulp stem cells were induced to differentiate into neural-like cells in each of the examples and comparative examples, which was consistent with the expression of specific proteins in neural-like cells. The expressio...
Embodiment 7
[0072] The cells were induced for 48 hours using the protocols of Comparative Example 1 and Example 1, and the cells were collected at 24 hours, 36 hours, and 48 hours of induction to extract total protein, and the expression levels of Nestin and MAP2 were detected by the method described in Example 6. The result is as Figure 6 . The results showed that after the cells were induced for 24 hours in Example 1, the expression levels of Nestin and MAP2 in the cells were significantly higher than those in the cells induced in Comparative Example 1 for 48 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com