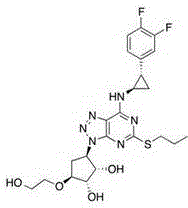
High performance liquid chromatography detection analysis method for controlling ticagrelor isomer
A high-performance liquid chromatography and ticagrelor technology, applied in the field of pharmaceutical chemical analysis, to achieve the effect of high chiral purity, product stability, and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Instruments and Conditions
[0026] High performance liquid chromatography: Shimadzu: LC-10ATvp, SPD-M10Avp;
[0027] Chromatographic column: OJ-H (DACEL4.6mm×250mm, 5μm);
[0028] Mobile phase: n-hexane – methanol = 80:20;
[0029] Flow rate: 1.0mL / min;
[0030] Column temperature: room temperature;
[0031] Injection volume: 10μL;
[0032] Detection wavelength: 225nm;
[0033] Experimental procedure
[0034] Weigh 5 mg of ticagrelor and its isomer II respectively, place in a 25 mL volumetric flask, add absolute ethanol
[0035] Dissolve and dilute to the mark, shake well, as a mixed sample solution. And weigh 5 mg of ticagrelor and its enantiomer II respectively, put them in two 25mL volumetric flasks, add absolute ethanol to dissolve and dilute to the scale, shake well, and use it as a qualitative control solution.
[0036] Take each control solution and mixed sample solution respectively, carry out high-performance liquid chromatography analysis according to...
Embodiment 2
[0039] Instruments and Conditions
[0040] High performance liquid chromatography: Shimadzu: LC-10ATvp, SPD-M10Avp;
[0041] Chromatographic column: OJ-H (DACEL4.6mm×250mm, 5μm);
[0042] Mobile phase: n-hexane – methanol = 85:15;
[0043] Flow rate: 1.0mL / min;
[0044] Column temperature: room temperature;
[0045] Injection volume: 10μL;
[0046] Detection wavelength: 225nm;
[0047] Experimental procedure
[0048] Weigh 5 mg of ticagrelor and its isomer II respectively, place in a 25 mL volumetric flask, add absolute ethanol
[0049] Dissolve and dilute to the mark, shake well, as a mixed sample solution. And weigh 5 mg of ticagrelor and its enantiomer II respectively, put them in two 25mL volumetric flasks, add absolute ethanol to dissolve and dilute to the scale, shake well, and use it as a qualitative control solution.
[0050] Take each control solution and mixed sample solution respectively, carry out high-performance liquid chromatography analysis according to...
Embodiment 3
[0053] Instruments and Conditions
[0054] High performance liquid chromatography: Shimadzu: LC-10ATvp, SPD-M10Avp;
[0055] Chromatographic column: OJ-H (DACEL4.6mm×250mm, 5μm);
[0056] Mobile phase: n-hexane – ethanol = 90:10;
[0057] Flow rate: 1.0mL / min;
[0058] Column temperature: room temperature;
[0059] Injection volume: 10μL;
[0060] Detection wavelength: 225nm;
[0061] Experimental procedure
[0062] Weigh 5 mg of ticagrelor and its isomer II respectively, place in a 25 mL volumetric flask, add absolute ethanol
[0063] Dissolve and dilute to the mark, shake well, as a mixed sample solution. And weigh 5 mg of ticagrelor and its enantiomer II respectively, put them in two 25mL volumetric flasks, add absolute ethanol to dissolve and dilute to the scale, shake well, and use it as a qualitative control solution.
[0064] Take each control solution and mixed sample solution respectively, carry out high-performance liquid chromatography analysis according to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com