Method for detecting ROS1 gene status based on rare cell and correlated kit
A technology of rare cells and kits, applied in the field of medical diagnostics, can solve problems such as inability to obtain materials repeatedly, failure to obtain materials, and inability to provide real-time monitoring information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Separation of blood samples
[0121] As shown in steps 1 to 6 of the above method of using the kit, test blood samples of 40 normal people, 40 cases of benign lung diseases, and 20 cases of lung cancer patients. The method is to add 3.2mL blood samples to a 50mL centrifuge tube and add CS1 to work. Centrifuge at 650×g for 5 minutes, and aspirate the supernatant; add CS2 working solution for lysis for 8 minutes, centrifuge at 650×g for 5 minutes, and aspirate the supernatant; add a certain amount of CS1 working solution again, and add 200uL of magnetic particle suspension , Shake for 20 minutes; pipette all the mixed liquid, superimpose it on the CS3 separation medium, centrifuge at 300×g for 5 minutes; pipette the liquid except the magnetic particle precipitation into a 15mL centrifuge tube, add CS1 working solution to 15mL, 950× Centrifuge for 5 minutes at g, aspirate and discard the supernatant; add 1 mL of CS1 working solution, mix by pipetting and add to a new 2 mL cen...
Embodiment 2
[0124] Preparation of the kit of the invention
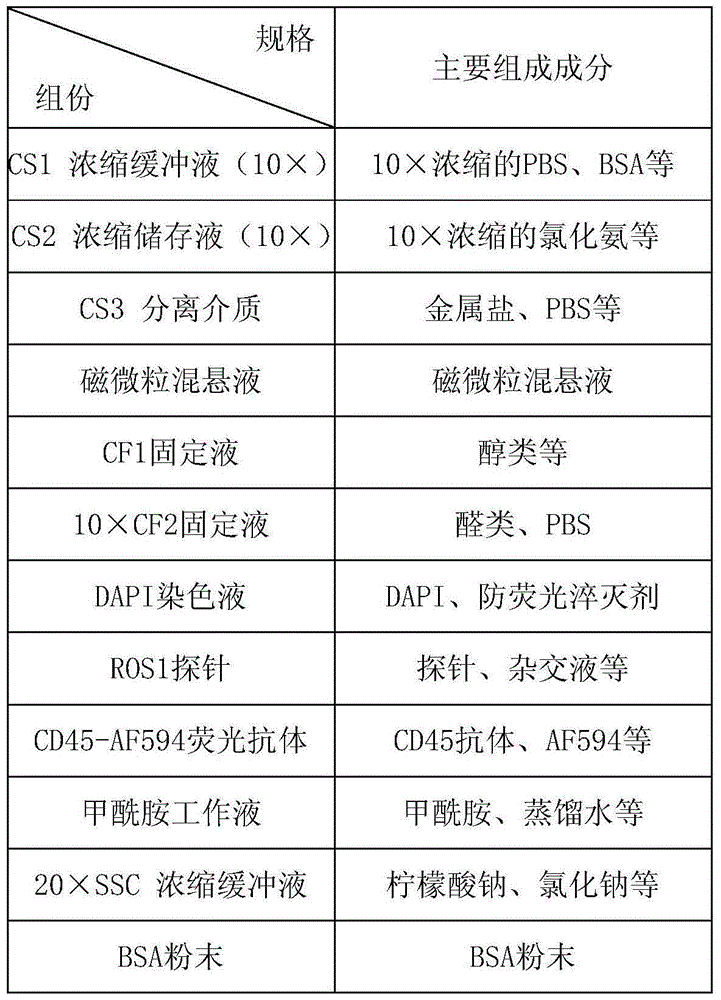
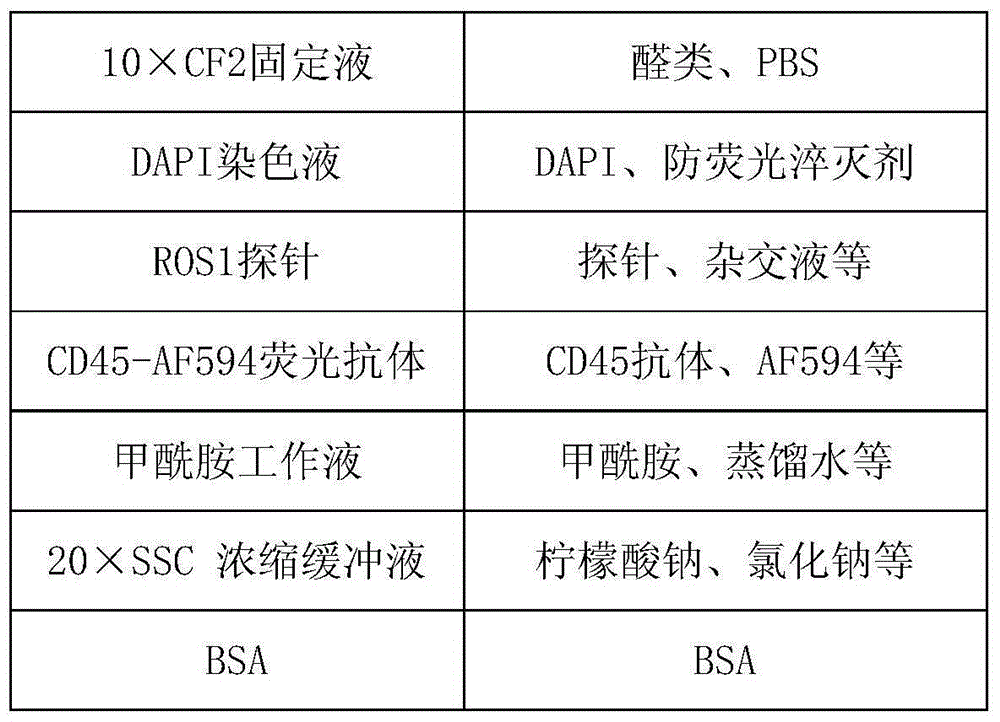
[0125] Kit one:
[0126] CS1 concentrated buffer (10×):
[0127] Each 1000mL water contains 60g BSA, 5 packets of PBS powder (2L / pack), 100mL 0.5M EDTA, 0.8mL Proclin300.
[0128] CS2 concentrated stock solution (10×):
[0129] Weigh 82.9gNH per 1000mL water 4 Cl, 10gKHCO 3 , 0.37g EDTA, water and 0.8mL Proclin300, stir enough to dissolve, make the volume constant, and prepare a 10X concentrate.
[0130] CS3 separation medium:
[0131] Dilute the gradient centrifugal liquid with a density of 1.077 and test the density during the dilution process to make the density between 1.070 and 1.075.
[0132] Magnetic particle suspension:
[0133] Adjust the CD45 antibody concentration to 1mg / mL, and incubate with streptavidin immunomagnetic beads at a ratio of 100uL:1mL for 1h to prepare a magnetic particle suspension.
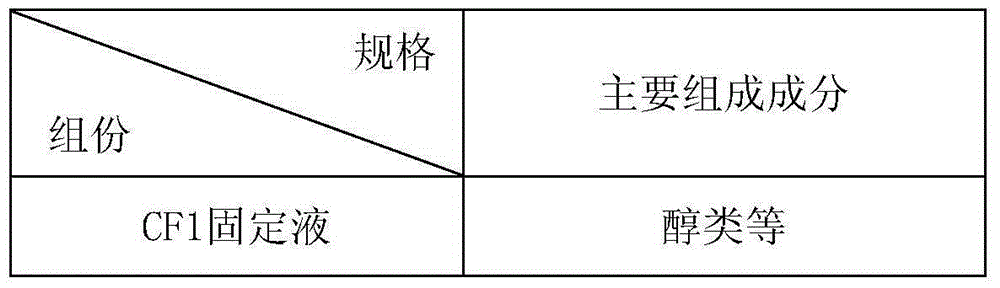
[0134] CF1 fixative:
[0135] Mix PEG and absolute ethanol so that the final concentration of PEG is 1% and the final concentration of ...
Embodiment 3
[0152] Use the kit of the present invention to detect other body fluid samples
[0153] Note: This kit is not only suitable for blood, but also for the detection of rare cells in other body fluids, such as pleural fluid, ascites, toilet fluid, amniotic fluid, etc., but not limited to these types of body fluids.
[0154] This kit was used to detect the pleural effusion of 6 cases of lung cancer, of which 1 case was found to have 2 positive ROS1 rearrangement cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com