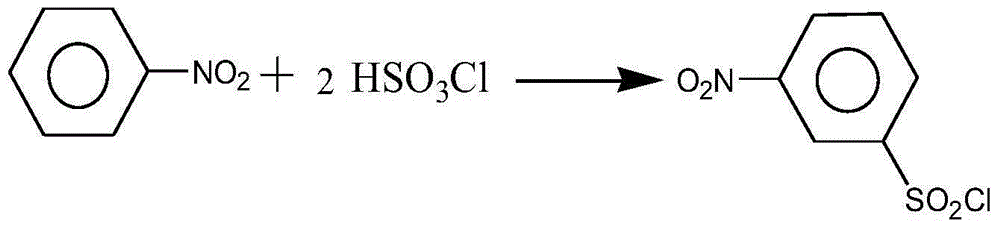Preparation method for aryl sulfonyl chloride derivative
A technology of arenesulfonyl chloride derivatives and products, which is applied in the field of preparation of arenesulfonyl chloride derivatives, can solve problems such as easy production of by-products, easy production of sulfur, and large amount of chlorosulfonic acid, so as to avoid pollution and ensure Purity, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of arene sulfonyl chloride derivatives, comprising the following steps:
[0030] 1) Preparation of chlorosulfonic acid: add a certain amount of hydrogen chloride gas and sulfur trioxide gas into the synthesizer, control the temperature at 115-130°C, cool after a period of reaction, and then separate to obtain chlorosulfonic acid for use;
[0031] 2) Preparation of crude aromatic alkyl sulfonyl chloride derivatives: add 2 to 6 moles of chlorosulfonic acid into the three-necked flask, and simultaneously feed 0.5 to 1.5 moles of sulfur trioxide gas to form a stable chlorosulfonic acid solution, and then pour the three-necked flask Slowly add 1 mole of aromatic hydrocarbon-based derivatives, and drop 1-2 drops of acetic acid at the same time, raise the temperature to 50-140 ° C, keep the temperature constant, react for 6-8 hours, and then lower the temperature in the three-necked flask to 40-60°C, add 0.5-3 moles of phosphorus oxychloride dropwise, then...
Embodiment 1
[0035] Add 233.04g of chlorosulfonic acid into a three-necked flask equipped with a thermometer and a stirrer, and introduce 40.03g of sulfur trioxide gas to form a stable chlorosulfonic acid solution, then slowly add 123.11g of nitrobenzene, and dropwise add 1 to 2 Drop acetic acid, raise the temperature to 50°C, stir and react at this temperature for 6h, after the end of the reaction, lower the temperature to 40°C, continue to drop 76.67g of phosphorus oxychloride into the three-necked flask, and raise the temperature to 60°C °C, react at this temperature for 2 hours, after the reaction is complete, lower the temperature to below 20 °C, and at the same time pass the hydrogen chloride gas generated by the reaction into water, the hydrogen chloride gas will be dissolved in water to generate hydrochloric acid as a by-product, and the crude aromatic hydrocarbon Acid chloride derivatives were added to ice water, stirred and diluted, and 1231.1g of carbon tetrachloride was added fo...
Embodiment 2
[0037] Add 699.12g of chlorosulfonic acid into a three-necked flask equipped with a thermometer and a stirrer, and introduce 120.09g of sulfur trioxide gas to form a stable chlorosulfonic acid solution, then slowly add 123.11g of nitrobenzene, and dropwise add 1 to 2 Drop acetic acid, raise the temperature to 50°C, stir and react at this temperature for 6h, after the end of the reaction, lower the temperature to 40°C, continue to drop 459.99g of phosphorus oxychloride into the three-necked flask, and raise the temperature to 60°C °C, react at this temperature for 2 hours, after the reaction is complete, lower the temperature to below 20 °C, and at the same time pass the hydrogen chloride gas generated by the reaction into water, the hydrogen chloride gas will be dissolved in water to generate hydrochloric acid as a by-product, and the crude aromatic hydrocarbon Acid chloride derivatives were added to ice water, stirred and diluted, and 1477.32g of carbon tetrachloride was added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com