Composition containing alogliptin and pioglitazone
A technology for pioglitazone and tablet cores, which is applied in the field of core-coated tablets containing alogliptin and pioglitazone, can solve the problems of inability to achieve blood sugar control, the proportion of drug-containing coating layers is large, and the production cost is increased, and the generation of by-products can be reduced. risks, ease of inspection and quality control, effectiveness of safety assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of the core-coated tablet of alogliptin and pioglitazone of the present invention comprises the following steps:
[0030] 1) Preparation of the chip: mix alogliptin (or pioglitazone) and auxiliary materials uniformly, press into tablets or make granules by conventional granulation means, and then press into tablets;
[0031] 2) Chip coating: the coating components can be applied to the chip by conventional coating techniques; such as using a film coating device and the like.
[0032] 3) Preparation of coated chips: mix pioglitazone (or alogliptin) with auxiliary materials uniformly or prepare granules by conventional granulation means, and then pressurize and wrap the prepared alogliptin in a core-coating tablet press. (or pioglitazone) coated chips.
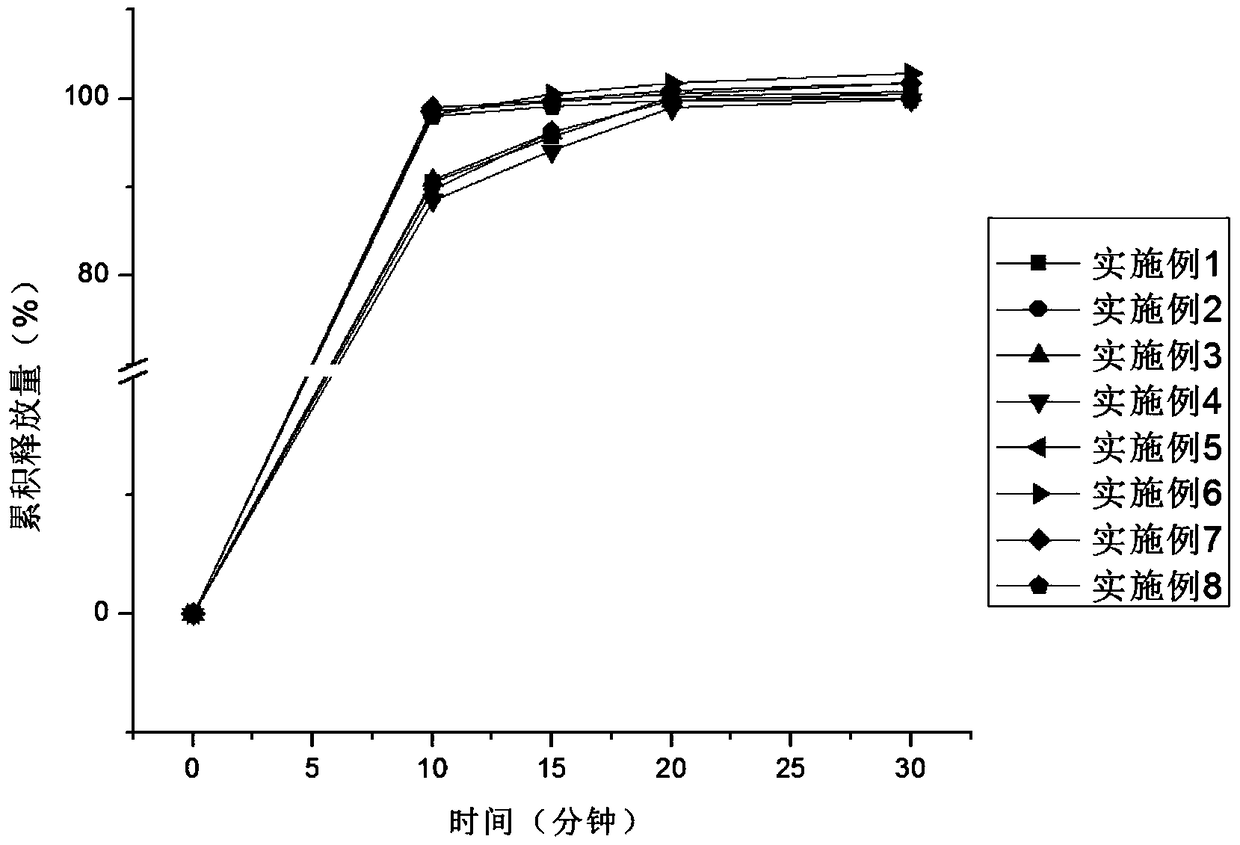
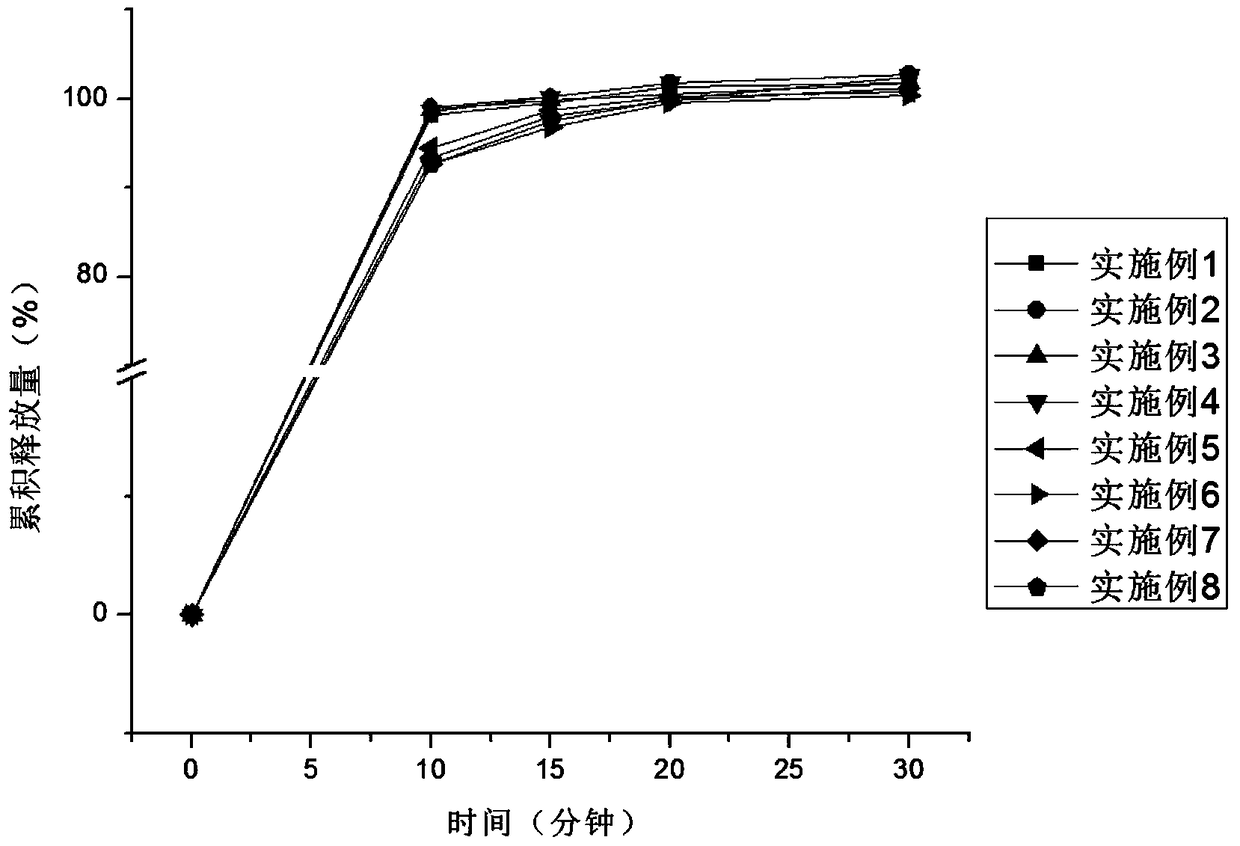
[0033] The core-coated tablet of alogliptin and pioglitazone provided by the invention has rapid dissolution in vitro, and the dissolution rate of each active component does not interfere with each...
Embodiment 1-4
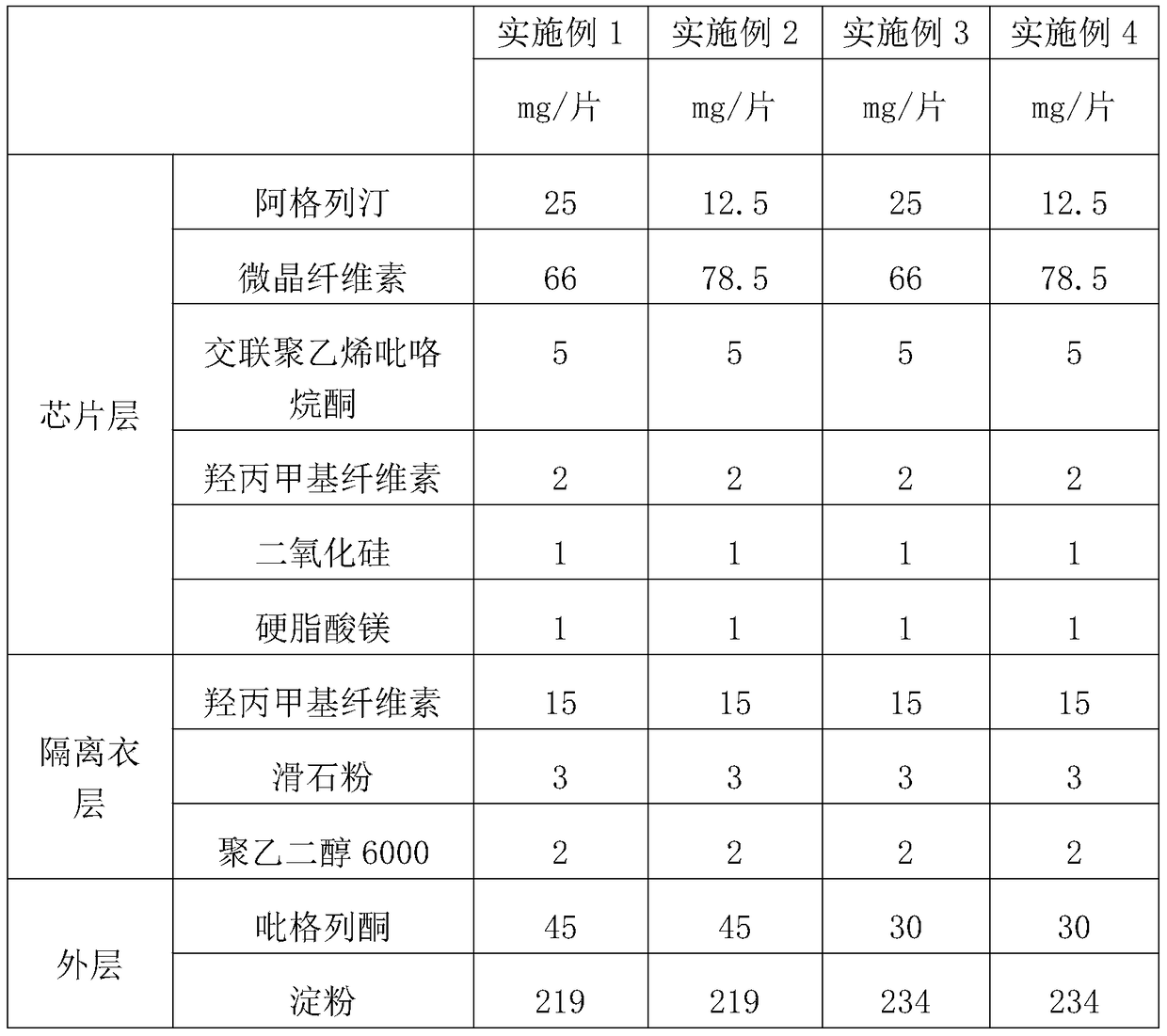
[0035] Table 1 embodiment 1-4 prepares the package chip of alogliptin and pioglitazone
[0036]
[0037]
[0038] According to the formula in Table 1, mix alogliptin with microcrystalline cellulose, cross-linked polyvinylpyrrolidone, 1 / 2 prescription amount of hydroxypropyl methylcellulose and silicon dioxide, dry granulate, and then add 1 / 2 prescription amount of hypromellose and magnesium stearate, mix well, and compress into tablets. The coating solution prepared according to the prescription is coated on the prepared alogliptin chip. According to the prescription, mix pioglitazone with starch, 1 / 2 prescription amount of sodium carboxymethyl starch, povidone and silicon dioxide, and then add 1 / 2 prescription amount of carboxymethyl starch sodium and stearic acid after dry granulation Magnesium, mixed evenly, and coated on the alogliptin coated chip by using a core-coated tablet press to obtain the coated chip of alogliptin and pioglitazone.
Embodiment 5-8
[0039] Table 2 embodiment 5-8 prepares the package chip of pioglitazone and alogliptin
[0040]
[0041]
[0042] According to the formula in Table 2, mix pioglitazone with starch, povidone, 1 / 2 prescription amount of sodium carboxymethyl starch and silicon dioxide, dry granulate, and then add 1 / 2 prescription amount of carboxymethyl starch Sodium and magnesium stearate, blended, compressed into tablets. The coating solution prepared according to the prescription is coated on the prepared pioglitazone chip. According to the prescription, mix alogliptin with microcrystalline cellulose, 1 / 2 prescription amount of hydroxypropyl methylcellulose, cross-linked polyvinylpyrrolidone and silicon dioxide, and then add 1 / 2 prescription amount Hypromellose and magnesium stearate are mixed evenly, and coated on the coated chips of pioglitazone by using a core-coated tablet press to obtain the coated chips of pioglitazone and alogliptin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com