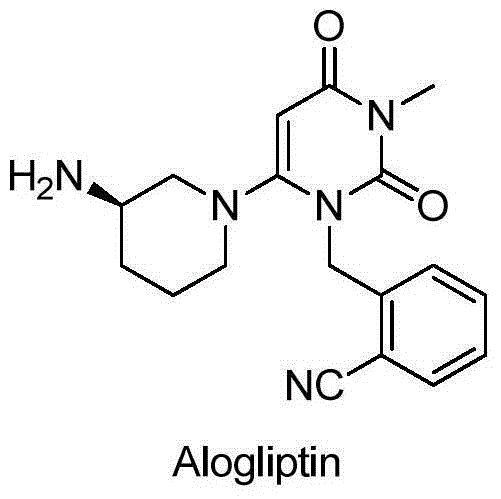
Method for preparing (R)-or(S)-3-aminopiperidine dihydrochloride
A technology of bishydrochloride and aminopiperidine, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as difficult realization and unguaranteed supply of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
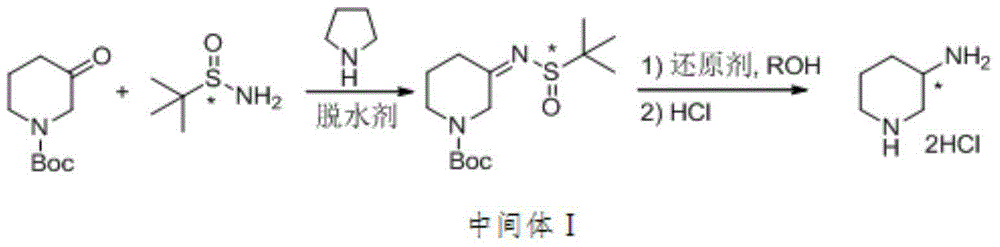
[0028] Synthesis of N-Boc-3-(R)-tert-butylsulfinimide piperidine:
[0029] Under the protection of nitrogen, add 220 mL of dichloromethane, (R)-tert-butylsulfinamide (13.3 g, 0.11 mol) and N-Boc-3-piperidine in sequence into a 500 mL reaction flask equipped with a mechanical stirring and reflux device Ketone (19.9g, 0.1mol), dissolved completely under stirring. Then 100 g of 4A molecular sieve and tetrahydropyrrole (0.71 g, 0.01 mol) were added, stirred at room temperature for 30 minutes, and then heated to reflux for 8 hours. TLC detected that the reaction did not change, and the reaction was stopped. After cooling, diatomaceous earth was filtered, and the filter cake was washed by adding 50 mL of dichloromethane. The filtrate was rotary evaporated to dryness with a water pump, and the obtained solid (25.3 g, crude product yield 84%) was recrystallized from n-hexane to obtain a solid product, and 160 mL of methanol was added to form a solution that was directly used in the n...
Embodiment 2
[0033] Synthesis of N-Boc-3-(S)-tert-butylsulfinimide piperidine:
[0034] Under nitrogen protection, 200 mL of tetrahydrofuran, (S)-tert-butylsulfinamide (18.2 g, 0.15 mol) and N-Boc-3-piperidone ( 19.9g, 0.1mol), completely dissolved under stirring. Then, 90 g of 5A molecular sieve and tetrahydropyrrole (0.71 g, 0.01 mol) were added, stirred at room temperature for 30 minutes, and then heated to reflux for 6 hours. TLC detected that the reaction was complete, and the reaction was stopped. After cooling, diatomaceous earth was filtered, and the filter cake was washed by adding 40 mL of tetrahydrofuran. The filtrate was rotary evaporated to dryness with a water pump, and the obtained solid (27.5 g, crude product yield 91%) was recrystallized from n-heptane to obtain a solid product, and 180 mL of ethanol was added to form a solution that was directly used in the next reaction.
[0035] Synthesis of (R)-3-aminopiperidine dihydrochloride:
[0036] Under the protection of nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com