Clinic-level adipose-derived stem cell preparation and storage methods
A technology of adipose stem cells and storage methods, which is applied in the field of preparation and storage of clinical-grade adipose stem cells, which can solve the problems of low yield rate of adipose tissue, ADSCs animal-derived protein pollution, and easy aging, so as to reduce the link of in vitro culture, Maintain cell phenotype and doubling time, plateau prolongation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
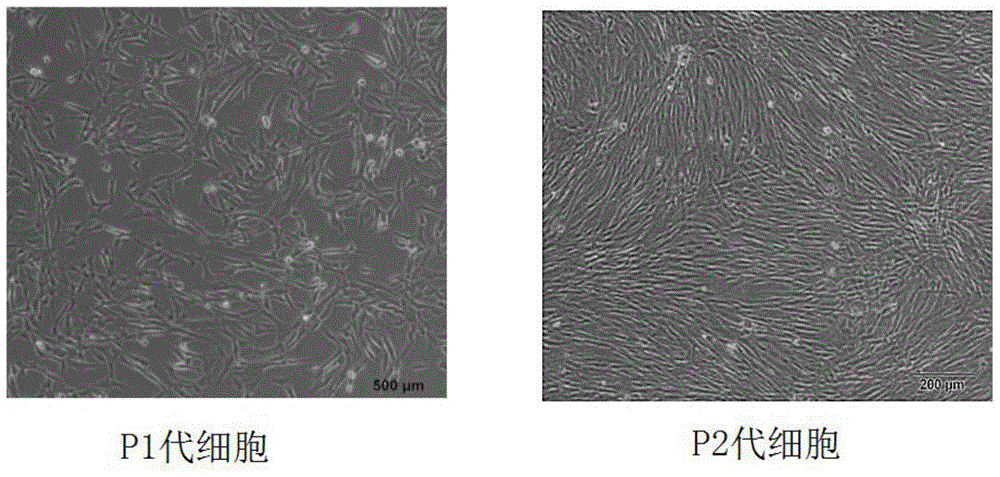
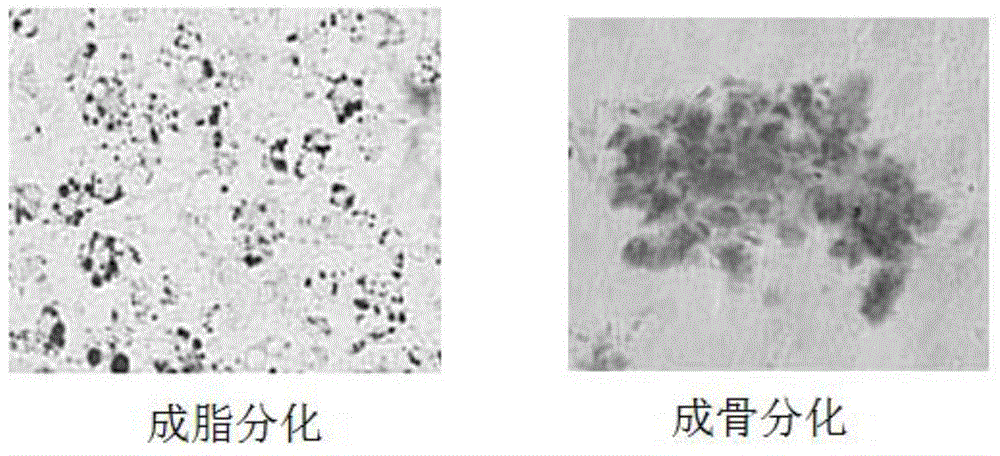
[0054] A method for preparing clinical grade adipose stem cells, comprising the following steps:
[0055] (1), the preparation of the platelet lysate PL of pathogen inactivation
[0056] Collect autologous platelets by machine or collect expired platelets from the central station, centrifuge at 20-25°C, 1200-1500rpm for 10-15min to inactivate pathogens;
[0057] Perform 3-5 consecutive cycles of -80°C freezing and 37°C recovery to fully lyse the platelets, centrifuge at 4000rpm for 30 minutes, collect the platelet lysate, freeze at -80°C for later use, and conduct pathogenic testing during the freezing period;
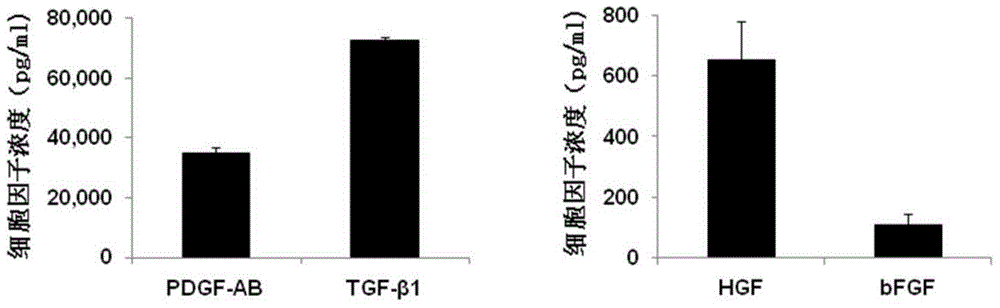
[0058] The content of cytokines in PL was measured by ELISA method, and the results were as follows: f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com