Growth matrices for stem cell propagation in vitro and in tissue regeneration
A stem cell and tissue technology, applied in the field of culturing stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 (stem cell morphology, proliferation and differentiation)
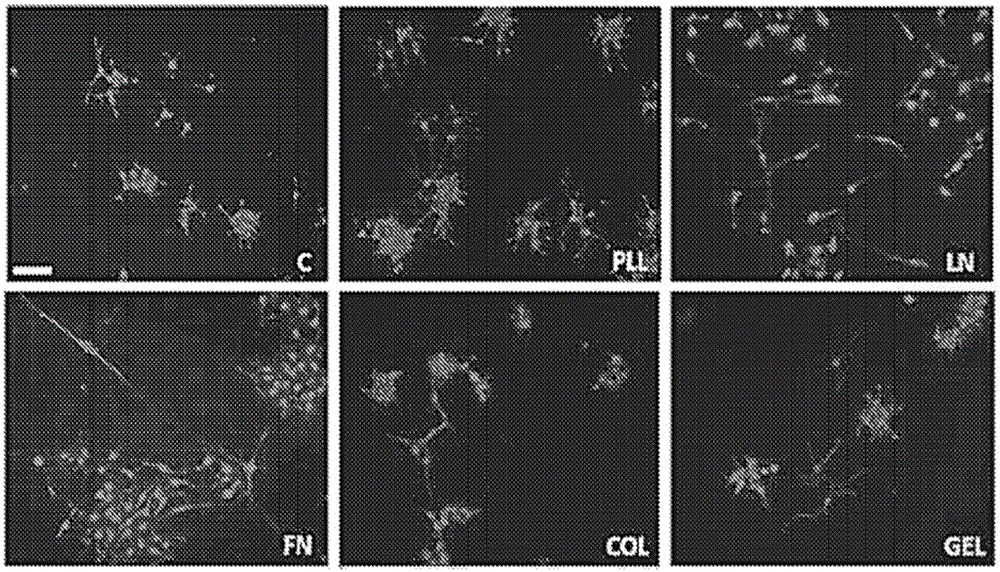
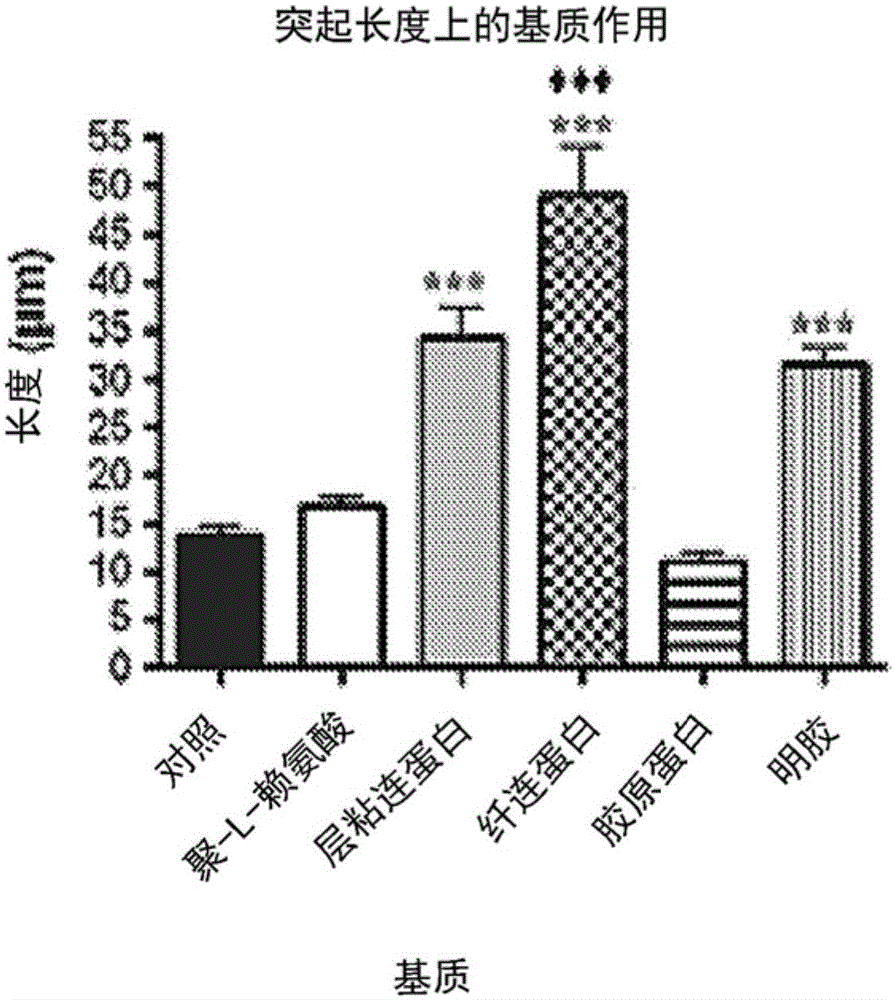
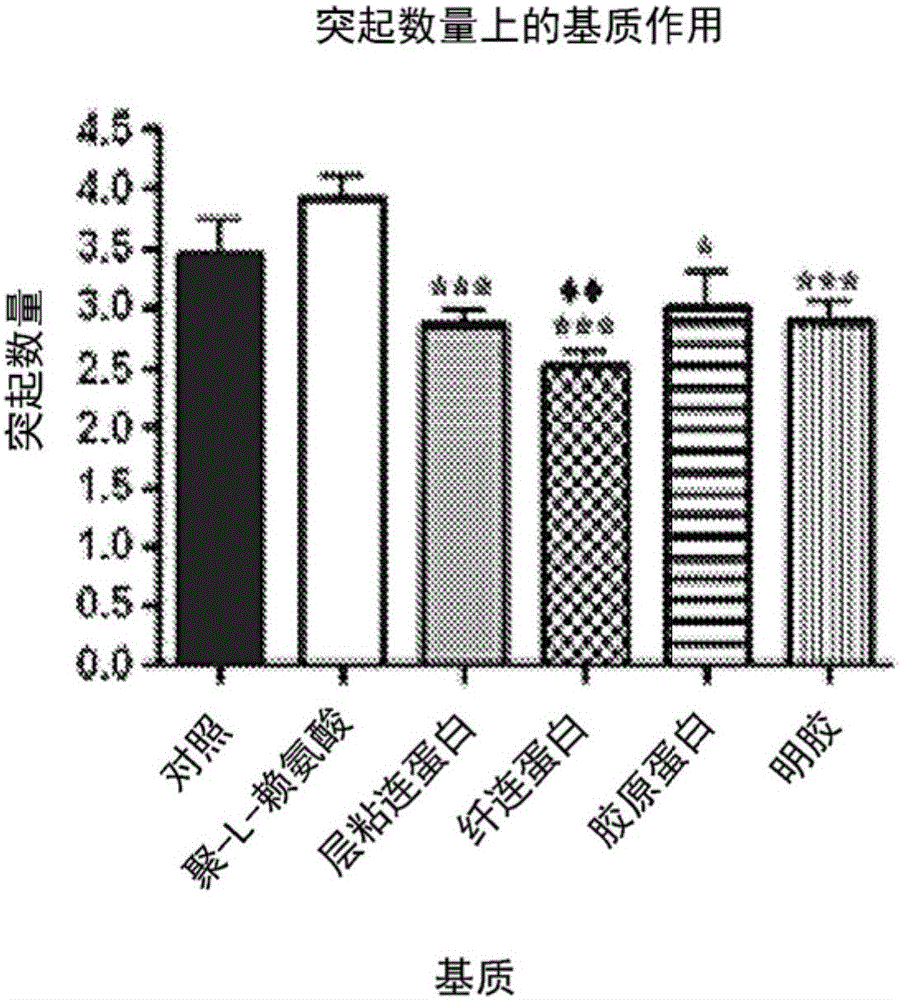
[0049] Neural stem cells: Two cell types were used to assess the functionality of modified chitosan membranes and microspheres: the immortalized cell line RG3.6, which is positive for green fluorescent protein (GFP) by day 13.5 embryos + ) from rat neocortical cells; and primary NSCs harvested from day 13.5 embryonic EGFP(SD-Tg(GFP)Bal / 2Rrrc(RRRC:0065)) rat neocortex. For specific experiments, the RG3.6 cell line was used instead of primary cells to eliminate multiple variables seen with heterogeneous primary cell cultures and to achieve greater consistency in matrix optimization. RG3.6 and primary NSCs were maintained supplemented with B27, gentamicin (50 μg / mL), apotransferrin (50 μg / mL), and rhFGF-2 (10 ng / mL + 1 ng / mL heparan sulfate ) in DMEM / F12 medium. They were grown as neurospheres or as attached monolayers on polyornithine / fibronectin-coated petri dishes.
[0050] Chitosan film: A 3% w...
Embodiment 2
[0055] Embodiment 2 (stent efficiency)
[0056] The biological activity of human fibroblast growth factor-2 (hFGF-2) bound to scaffolds was verified by measuring cell growth using MTT assay and analyzing the morphology of NSCs. Heparin sodium salt from bovine intestinal mucosa was purchased from Sigma (St Louis, MO). Recombinant human fibroblast growth factor-2 (rhFGF-2) was purchased from Peprotech (Rocky Hill, NJ). Genipin was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
[0057] Cell Growth: Coat 96-well plates with 50 μL of 3% chitosan and air dry overnight. Selection coated wells were incubated with heparin (0.5 mg / mL) and genipin (0.45 mM) overnight at room temperature. Heparin-genipin solutions were prepared in 50 mM HEPES + 0.9% NaCl solution (HBS) (VWR; West Chester, PA). Other wells were incubated overnight in HBS only. The following day, fibronectin (10 ug / mL) was added to each well for 4 hours at 37°C to enhance cell attachment to the chi...
Embodiment 3
[0063] Embodiment 3 (characterization of 3-dimensional support)
[0064] Preparation of chitosan microspheres: Chitosan powder (1.5 g) was dispersed in 50 mL of water containing 2.0% v / v acetic acid to create a 3% chitosan solution. The chitosan solution was stirred mechanically at 700 rpm until completely dissolved. The resulting solution was collected and centrifuged at 2,000 rpm for 10 minutes. Subsequently, the supernatant was collected and the remaining impurities precipitated were discarded. Squeeze the acidic chitosan solution into an alkaline coagulation bath (composed of 2.5 M NaOH:methanol:water (20:30:50 v / v)) using a syringe at a flow rate of 5 mL / hr to form chitosan Microspheres. In order to reduce the surface tension on the needle tip, thereby reducing the size of the microspheres to the desired range, a current of 25 kV was applied. Next, filter the spheres using a 100 μm filter to remove any oversized spheres. Remove it from the ionic solution and rinse 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com