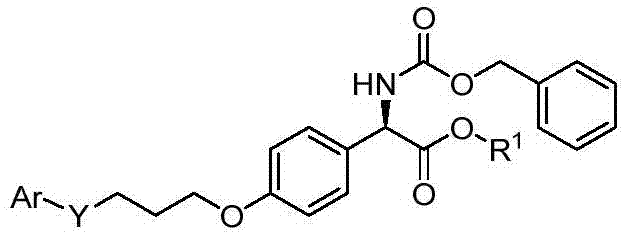
D-p-hydroxyphenylglycine derivatives and their preparation methods and applications
A technology of p-hydroxyphenylglycine and derivatives, which is applied in the field of drug synthesis and can solve the problem of less research on D-p-hydroxyphenylglycine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
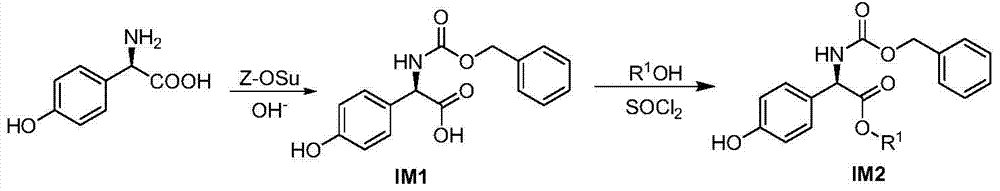
[0096] The synthesis of embodiment 1 intermediate IM1 and IM2
[0097]
[0098] In a 2L round bottom flask, add SM 83.55g (499.84mmol) and 2mol / L K 2 CO 3 300mL aqueous solution, stirred and dissolved under ice-bath cooling, then added 200mL of Z-OSu 130.11g (522.07mmol) acetone solution, and used 2mol / L K 2 CO 3 Adjust the pH of the aqueous solution to 8-9, stir and react at ambient temperature (15-35° C.), keep the pH constant during the reaction, and monitor the reaction progress by thin-layer chromatography (TLC). After the reaction is over, adjust pH=7 with 3mol / L hydrochloric acid, remove acetone by rotary evaporation, and saturate K 2 CO 3 The aqueous solution was adjusted to pH=9, ethyl acetate (EtOAc) was added for extraction (2×150 mL), the aqueous phase was cooled in an ice bath, concentrated hydrochloric acid was adjusted to pH=3-4, a large amount of solids precipitated, and EtOAc was added for extraction (3×250 mL), collected and The organic phases were co...
Embodiment 2
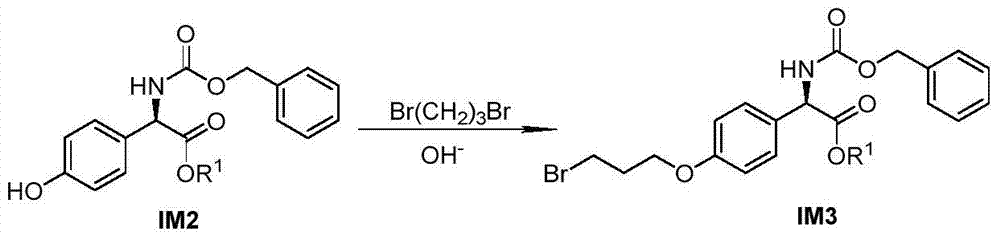
[0107] The synthesis of embodiment 2 intermediate IM3
[0108]
[0109] Add appropriate amount of IM2, acetonitrile, finely ground anhydrous K to a round bottom flask of appropriate size 2 CO 3 , stirred, and then added 1,3-dibromopropane (IM2:K 2 CO 3 :1,3-dibromopropane molar ratio is 1:4:6), heated in an oil bath at 50°C, and monitored the reaction progress by TLC. After the reaction, suction filtration and rotary evaporation of the filtrate gave a brownish-yellow oily product. Silica gel column chromatography, the eluent was concentrated by rotary evaporation, natural cooling for crystallization, suction filtration, and vacuum drying to obtain the intermediate IM3.
[0110] Table 2 Synthetic data of intermediate IM3
[0111]
[0112] IM3-a (R)-methyl 2-(((benzyloxy)carbonyl)amino)-2-(4-(3-bromopropoxy)phenyl)acetate: white solid; m.p.: 84-85°C ; 1 H NMR (600MHz, DMSO-d 6 )δ:2.21-2.25(m,2H,-CH 2 -),3.61(s,3H,OCH 3 ),3.66(t,2H,J=6.6Hz,-CH 2 Br), 4.07(t, 2H, J...
Embodiment 3
[0116] The synthesis of embodiment 3 compound TM1
[0117]
[0118] Add aromatic compound A and 5-15mL N,N-dimethylformamide (DMF) into a round bottom flask, stir to dissolve, then add powdered K 2 CO 3 After stirring for 30 minutes, IM3 was added, and the reaction was stirred at ambient temperature (15-35° C.), and the progress of the reaction was monitored by TLC. After the reaction, pour into ice water, extract with EtOAc, collect the organic phase, wash with water, anhydrous Na 2 SO 4 Dry, filter with suction, concentrate the filtrate by rotary evaporation under reduced pressure, and perform silica gel column chromatography to obtain the target compound TM1.
[0119] Table 3 Synthetic data of compound TM1
[0120]
[0121]
[0122]
[0123]
[0124]
[0125]
[0126] TM1-1a (R)-methyl 2-((benzyloxycarbonyl)amino)-2-(4-(3-(3-formylphenoxy)propoxy)phenyl)acetate: white solid; m.p. :87-88℃; 1 H NMR (300MHz, CDCl 3 )δ: 9.97 (1H, s, CHO), 7.17-7.46 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com