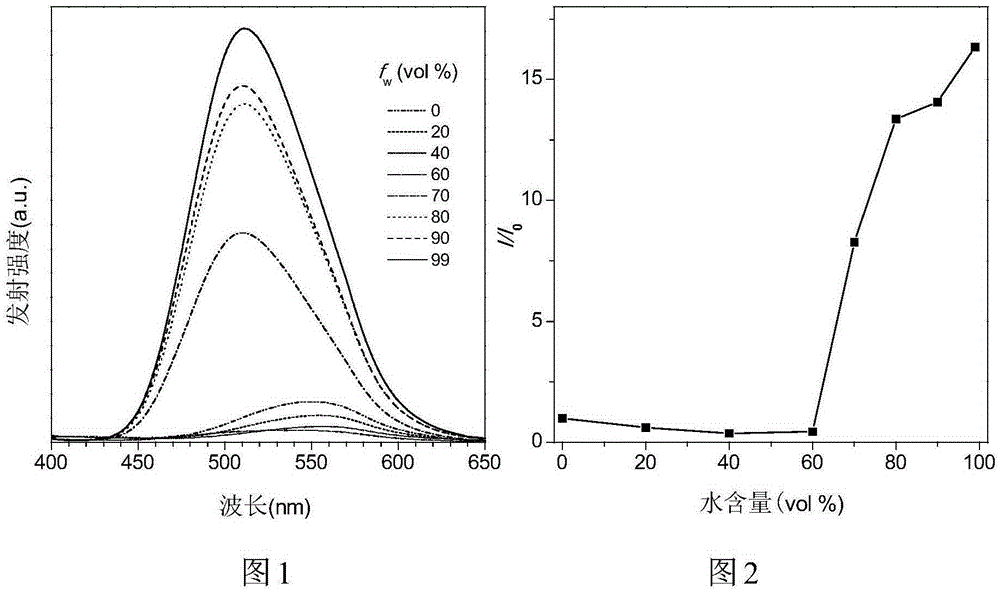
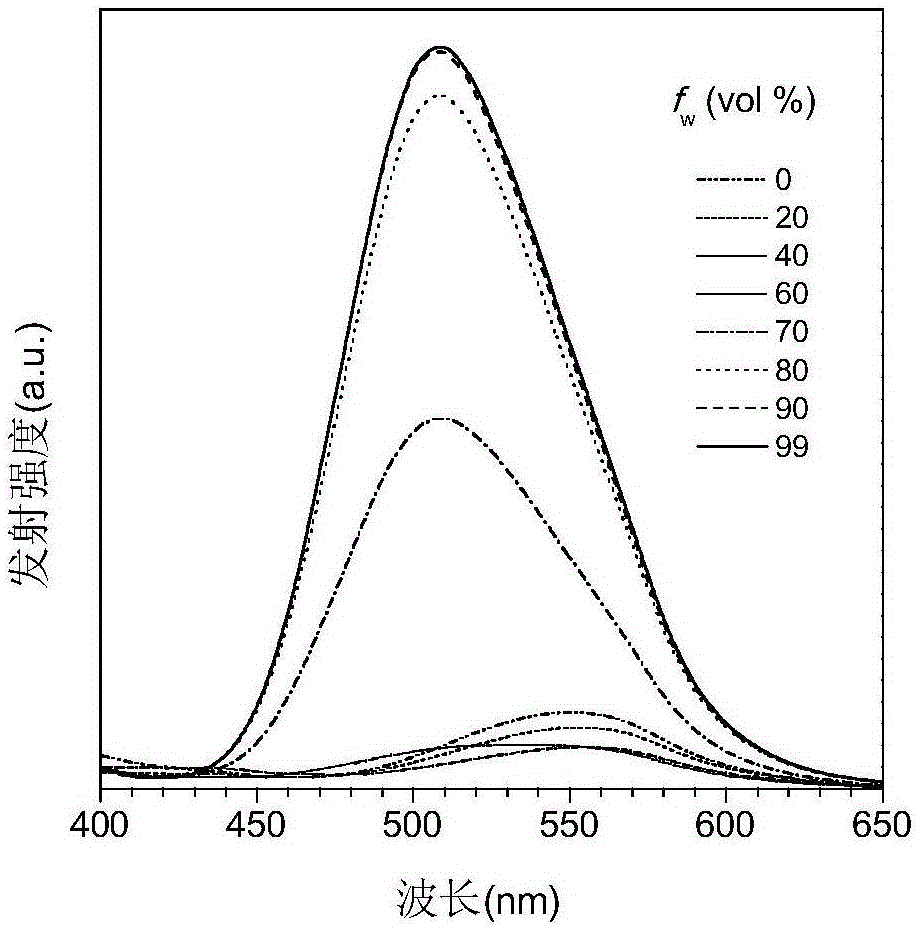
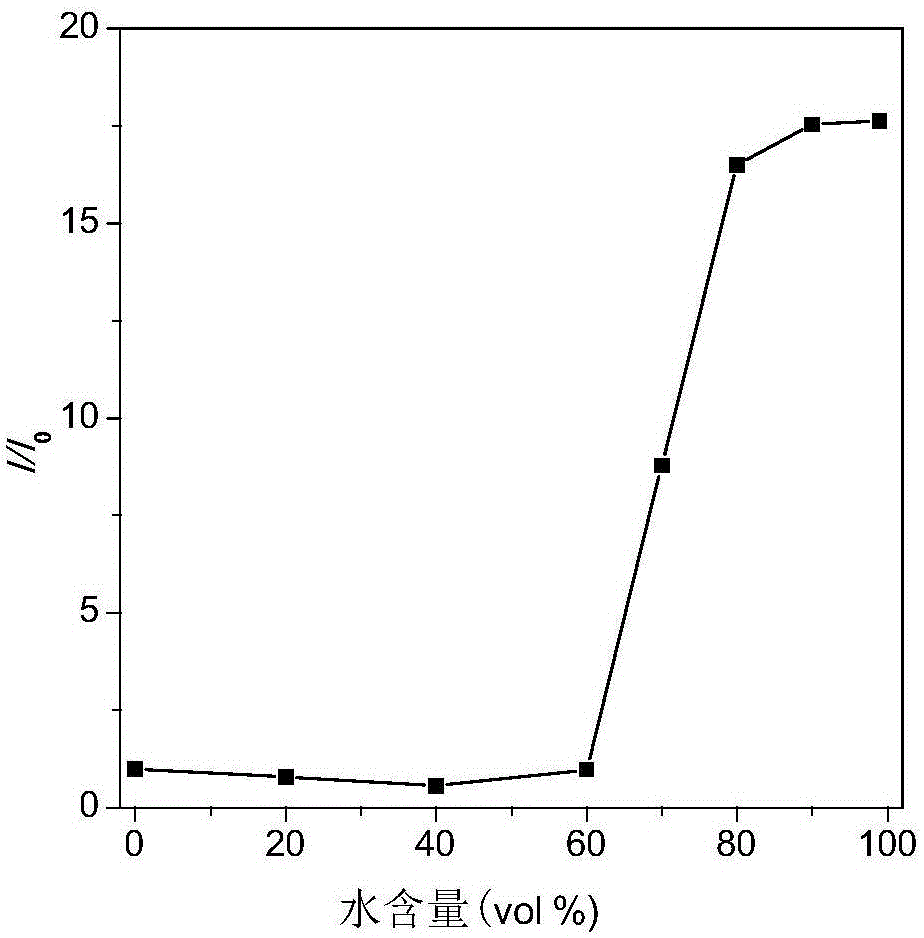
Binaphthol-based aggregation-induced light-emitting chiral fluorescent material
A technology for aggregation-induced luminescence and fluorescent materials, which is applied in the field of aggregation-induced luminescence chiral fluorescent materials to achieve good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Synthesis of BINOL-CHO
[0036]
[0037]Compound 1 (430 mg, 0.698 mmol) was dissolved in 6 mL THF under ice-water bath conditions, and then 3 mL concentrated hydrochloric acid was slowly added to the above solution. Then the mixture was allowed to warm to room temperature naturally and stirred for 3 hours. After the reaction was complete, the mixture was extracted with ethyl acetate (4×20 mL). The combined organic phases were washed successively with water, saturated sodium bicarbonate and saturated brine, and dried over anhydrous sodium sulfate. Subsequently, the solvent was evaporated under reduced pressure to obtain the product BINOL-CHO with a yield of 91%, and the structural characterization data were: 1 HNMR (400MHz, CDCl 3 , δ): 10.60 (s, 2H, CHO), 10.18 (s, 2H, OH), 8.34 (s, 2H, ArH), 8.00-7.98 (m, 2H, ArH), 7.44-7.38 (m, 4H, ArH), 7.22-7.20 (m, 2H, ArH); 13 CNMR (75MHz, CDCl 3 , δ): 196.92, 153.78, 138.65, 137.57, 130.83, 130.15, 127.78, 124.99, 124...
Embodiment 2
[0045] Synthesis of BINOM-CN
[0046]
[0047] Compound 1 (129 mg, 0.3 mol) was dissolved in 5 mL of ethanol, then malononitrile (40.6 mg, 0.615 mmol) and a drop of 1 mol / L NaOH aqueous solution were added, and stirred at room temperature for 2.5 hours. After the reaction was completed, the reactant was filtered under reduced pressure to obtain the target compound BINOM-CN with a yield of 46%, and the structural characterization data were: 1 HNMR (300MHz, CDCl 3 , δ): 8.94 (s, 2H, CH), 8.45 (s, 2H, ArH), 8.08 (d, J=8.0Hz, 2H, ArH), 7..60-7.55 (m, 2H, ArH), 7.50-7.45(m, 2H, ArH), 7.16(d, J=8.4Hz, 2H, ArH), 4.56(d, J=8.0Hz, 2H, CH 2 ), 4.50 (d, J=8.0Hz, 2H, CH 2 ), 3.09 (s, 6H, CH 3 ); 13 CNMR (75MHz, CDCl 3 , δ): 156.49, 152.26, 136.39, 131.78, 130.66, 130.30, 130.19, 127.14, 125.93, 125.61, 125.38, 113.85, 112.79, 100.68, 83.94, 57.50; ]+C 32 h 22 N 4 o 4 The theoretical value of Na is 549.1539, and the measured value is 549.1508.
Embodiment 3
[0049] Synthesis of BINOM-CN-Ph
[0050]
[0051] Compound 1 (108.5 mg, 0.25 mmol) was dissolved in 4 mL of ethanol, then phenylacetonitrile (58 μL, 0.5 mmol) and a drop of 1 mol / L NaOH aqueous solution were added, and stirred at room temperature for 2.5 hours. After the reaction was completed, the reactant was evaporated to remove the solvent under reduced pressure, and purified by column chromatography (petroleum ether / ethyl acetate=15:1) to obtain the target compound BINOM-CN-Ph, the yield was 53%, and the structural characterization data was : 1 HNMR (600MHz, CDCl 3 , δ): 8.86 (s, 2H, CH), 8.22 (s, 2H, ArH), 8.07 (d, J=6.0Hz, 2H, ArH), 7.79 (d, J=6.0Hz, 4H, ArH), 7.45-7.55(m, 6H, ArH), 7.35-7.43(m, 4H, ArH), 7.30(d, J=6.0Hz, 2H, ArH), 4.66(d, J=6.0Hz, 2H, CH 2 ), 4.62 (d, J=6.0Hz, 2H, CH 2 ), 2.80 (s, 6H, CH 3 ); 13 C-NMR (75MHz, CDCl 3 , δ): 152.22, 138.13, 134.82, 134.36, 130.64, 130.03, 129.55, 129.28, 128.35, 127.98, 126.16, 125.67, 117.99, 113.64, 100.15, 57...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com