Catalyst for hydrogen production by ethanol steam reforming and preparation method of catalyst
A steam reforming and catalyst technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., can solve problems that need to be improved urgently, achieve selectivity and operational stability and stability, less by-products, and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
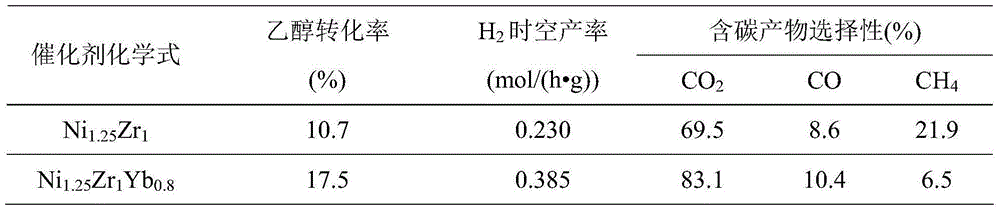
[0023] Mix 7.27g of nickel nitrate, 7.86g of zirconium nitrate and 7.47g of ytterbium nitrate (all of which are of AR grade), and add 100mL of deionized water to make solution A; another 12.30g (AR grade) of anhydrous K 2 CO 3 Dissolve in 100 mL deionized water to make solution B. At a temperature of 80°C, simultaneously inject solution A and solution B into a 500mL beaker preloaded with 200mL deionized water (injection rate is about 18mL / min), and carry out co-precipitation reaction at a constant temperature of 80°C and constant stirring. The pH value of the precipitation solution is maintained at 7-8 by adjusting the addition amount of solution A and solution B. After the addition, continue to stir for 30 minutes, then filter the feed liquid, the obtained precipitate is washed with deionized water several times and then filtered, the obtained solid is dried at 110°C for 10 hours, and roasted at 400°C for 4 hours to obtain the ethanol water to be prepared Steam reforming ca...
Embodiment 2
[0031] Mix 5.82g of cobalt nitrate, 2.97g of zinc nitrate and 1.02g of scandium nitrate (all of which are of AR grade), and add 100mL of deionized water to make solution A; another 4.77g (AR grade) of anhydrous K 2 CO 3 Dissolve in 100 mL deionized water to make solution B. At a temperature of 80°C, simultaneously inject solution A and solution B into a 500mL beaker preloaded with 200mL deionized water (injection rate is about 25mL / min), and carry out co-precipitation reaction at a constant temperature of 80°C and constant stirring. The pH value of the precipitation solution is maintained at 7-8 by adjusting the addition amount of solution A and solution B. After the addition, continue to stir for 30 minutes, and then filter the feed liquid. The obtained precipitate is washed with deionized water several times and then filtered. The obtained solid is dried at 110°C for 12 hours, and roasted at 350°C for 2 hours to obtain the ethanol water to be prepared. Steam reforming cata...
Embodiment 3
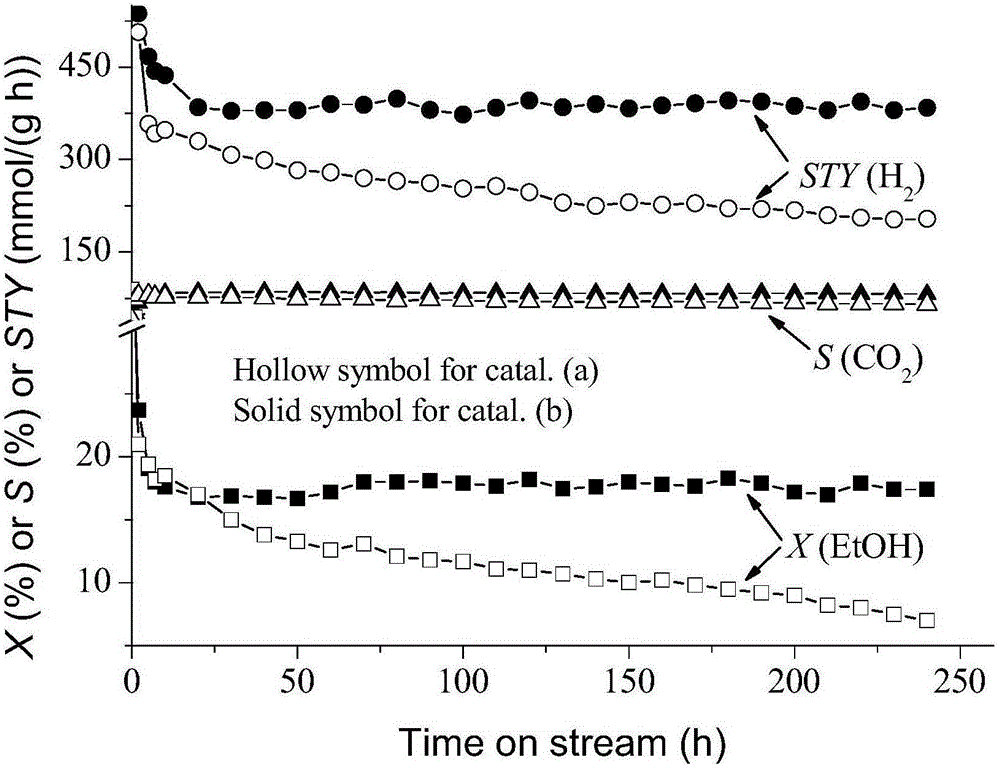
[0034] Keep the catalyst and activity evaluation experimental device in Example 2. The evaluation results show that at 0.5MPa and 450°C, the feed gas composition is CH 3 CH 2 OH / H 2 O / N 2 =10 / 30 / 60 (molar ratio), under the reaction conditions of space velocity GHSV=150000mL / (h g), the conversion rate of ethanol and the corresponding H 2 The space-time yields reached 22.0% and 0.909mol / (h g), respectively, CO 2 , CO and CH 4 The selectivities are 81.9%, 8.9% and 9.2%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com