Flounder neuropeptide and its application
A neuropeptide and flounder technology, which is applied in the field of protein expression application, can solve the problems such as the lack of biological activity of proteins and the inability of short peptides to exert their activities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Screening and sequence analysis of flounder neuropeptides
[0021] 1. Screening of flounder neuropeptides
[0022] The applicant conducted sequence analysis on the flounder genome library data obtained by our laboratory to explore the nucleotide sequence encoding small peptides, and then performed BLAST comparison on the encoded amino acid sequences to screen out the neuroendocrine system-related proteins of interest. Through screening, we found a nucleotide whose sequence is SEQ ID NO: 2, which can encode a small protein with an amino acid sequence of SEQ ID NO: 1. The blastp analysis of the protein containing 127 amino acids found that it is consistent with NCBI Compared with those reported in , the similarity was up to 78%. It was subsequently named flounder neuropeptide according to its function.
Embodiment 2
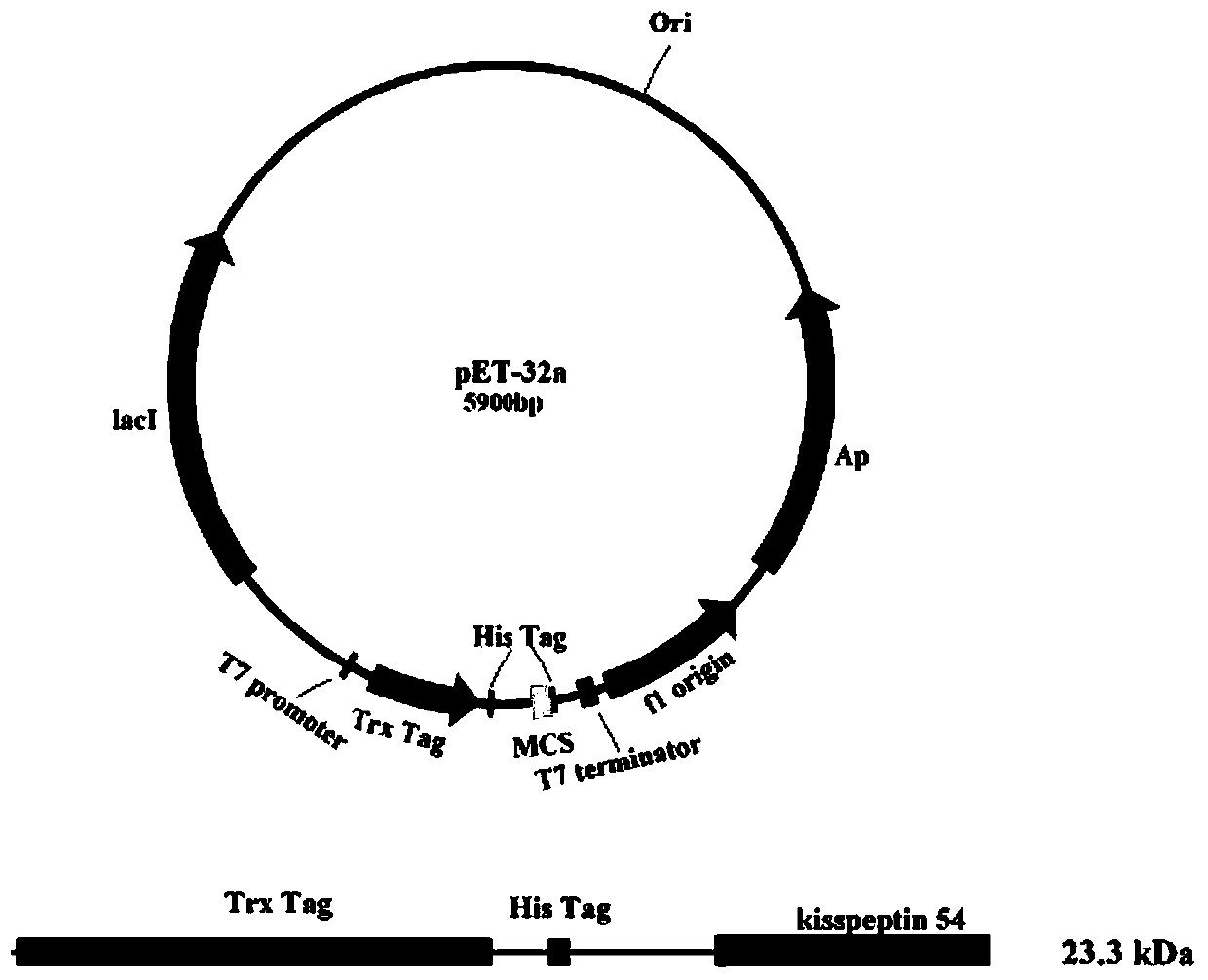
[0023] Example 2 Construction of recombinant expression vector, identification and expression of neuropeptide
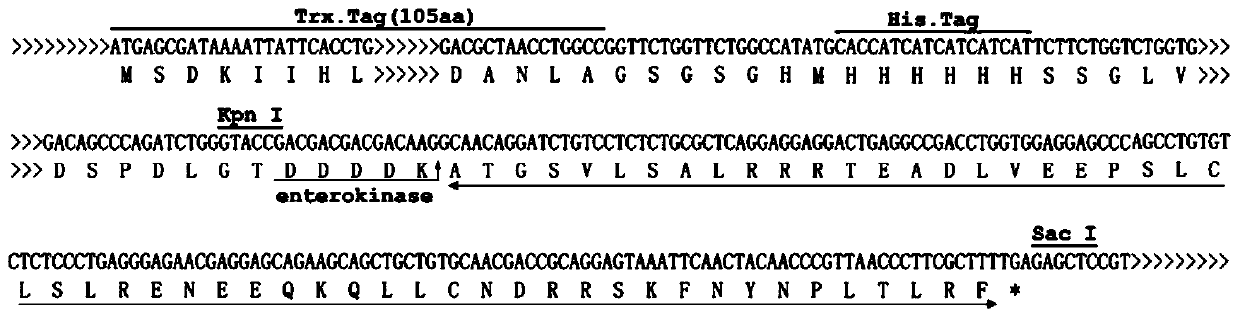
[0024] Construction and identification of the recombinant plasmid: According to the cDNA sequence of the flounder neuropeptide (SEQ ID NO: 2), a pair of specific primers P1 and P2 were used to amplify the coding sequence of the flounder neuropeptide. A restriction endonuclease site Kpn I and a sequence encoding an enterokinase cleavage site are introduced at the 5' end of the P1 primer, and a restriction endonuclease site Sac I and a stop codon coding are introduced at the 5' end of the P2 primer Sequence TCA, the sequences of the two primers are:
[0025] P1: GGTACCGACGACGACGACAAGGCAACAGGATCTGTCCTCTCTGC
[0026] P2: GAGCTCTCAAAAGCGAAGGGTTAACGGGTTGTAGTTG;
[0027] The PCR reaction volume was 25ul, and the amplification conditions were: 94°C for 5min; 35 cycles of 94°C for 30s, 68°C for 30s, and 72°C for 30s; 72°C for 7min. After the PCR product was detected by aga...
Embodiment 3
[0028] Example 3 Expression, purification and functional verification of recombinantly expressed flounder neuropeptide
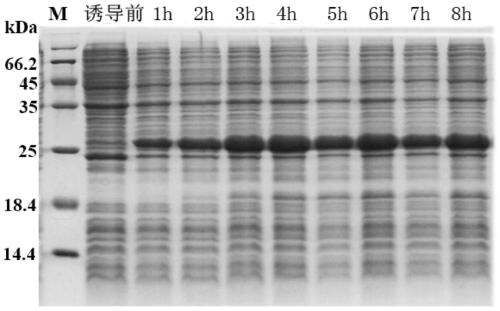
[0029] 1. Induced expression of recombinant protein:
[0030] The recombinant vector obtained above was introduced into the host strain Transetta (DE3), and the LB medium containing 100ug / mL ampicillin and 34ug / mL chloramphenicol was used for expansion culture, and PCR reaction was carried out with P1 and P2 as primers to screen the transformed into Correctly recombine the engineered strain of the expression vector and perform sequencing to verify that no base mutation occurs.
[0031] Inoculate the single clone of the screened engineered strain in 2×YT culture medium (containing 100ug / mL ampicillin and 34ug / mL chloramphenicol) at 37°C to expand the culture to OD 600 At about 0.6, add the inducer isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 1mM, induce expression at 37°C, and take samples at intervals of 1 hour, and collect the cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com