Electrochromic material and preparation method thereof
An electrochromic material and electrode technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of high oxidation potential, poor mechanical properties, and large energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Put the Ag wire in a hydrochloric acid solution with a concentration of 4mol / L, and after electrolysis at a constant potential of 1.7V for 100s, a red AgCl adhesion layer is formed on the surface as a reference electrode. The ITO glass electrode was wiped clean with alcohol and used as the working electrode. The stainless steel electrode was polished with 1200-mesh sandpaper, rinsed with water, ethanol, and acetone in sequence, and dried to serve as a counter electrode.
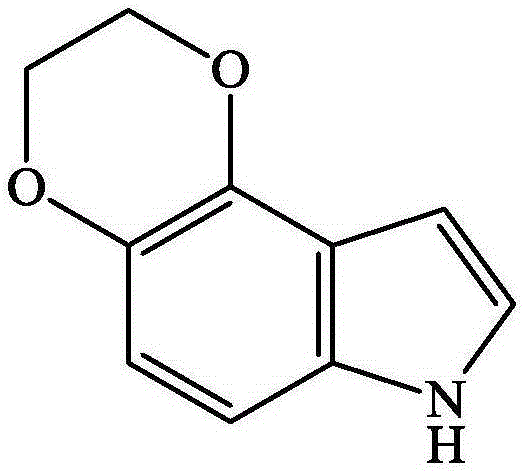
[0020] (2) Weigh 0.0175 g of solid 4,5-dioxyethylene indole and dissolve it in 5 mL of boron trifluoride ether solution, and configure the reaction of 4,5-dioxyethylene indole monomer concentration of 0.02 mol / L solution. Combine the three electrodes in step (1), put them into the boron trifluoride ether solution containing 4,5-dioxethyleneindole monomer, apply a voltage of 0.5V.vs.SCE, and react for 50s.
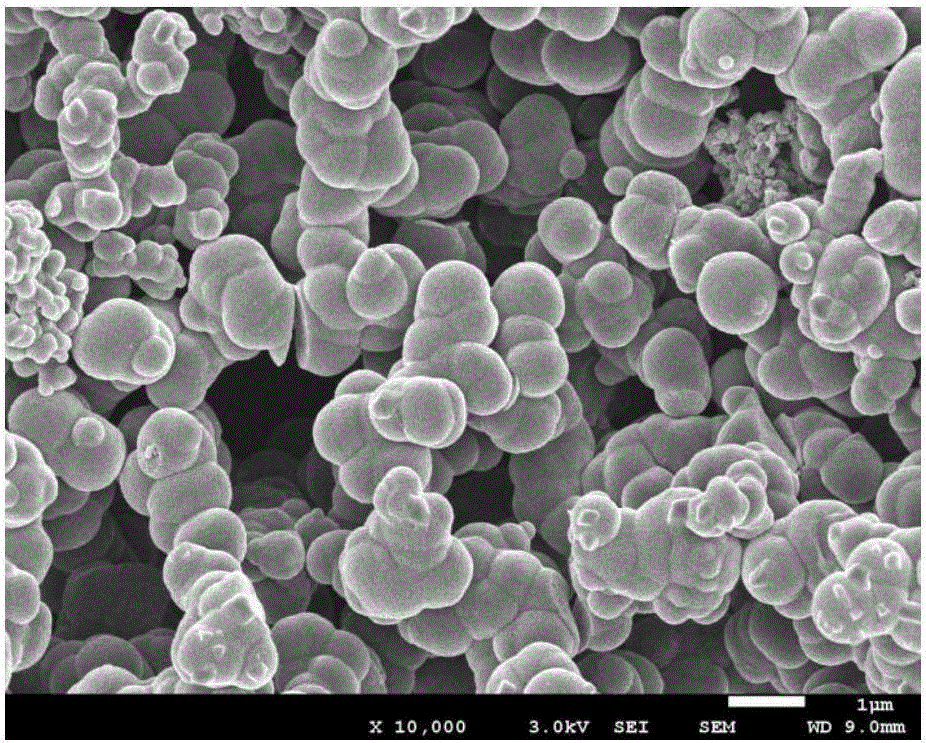
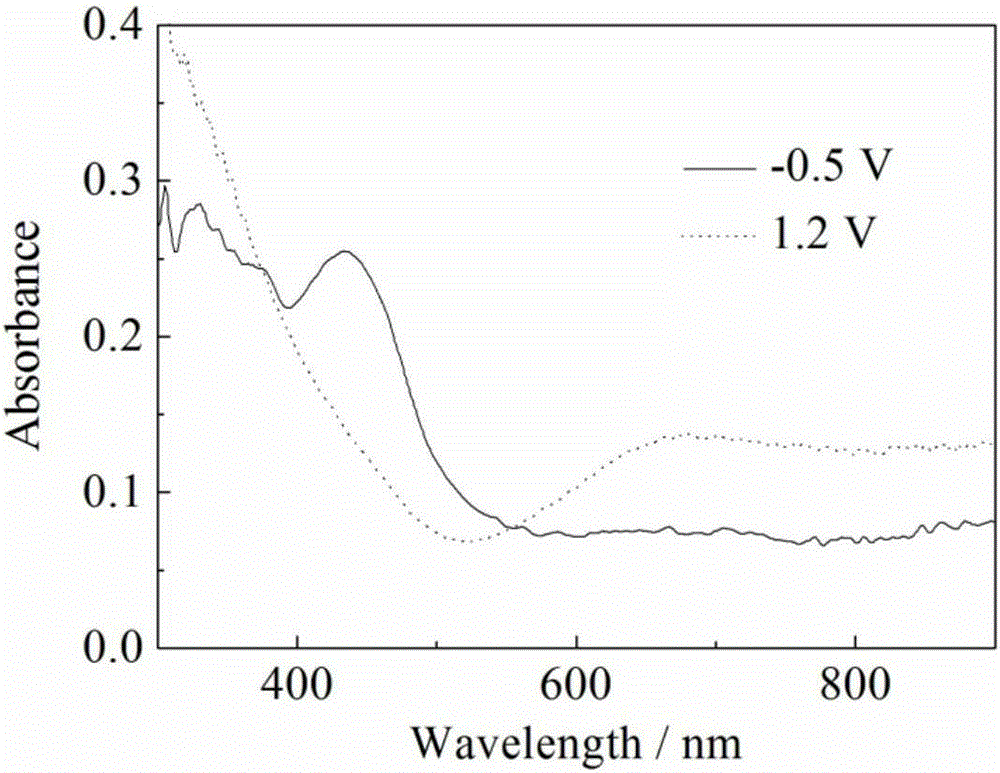
[0021] (3) The working electrode in step (2) is taken out, and the poly(4,5-dioxyethylene ...
Embodiment 2
[0023] (1) Put the Ag wire in a hydrochloric acid solution with a concentration of 8mol / L, and after electrolysis at a constant potential of 1.4V for 60s, a red AgCl adhesion layer is formed on the surface as a reference electrode. The ITO glass electrode was wiped clean with alcohol and used as the working electrode. The stainless steel electrode was polished with 1200-mesh sandpaper, rinsed with water, ethanol, and acetone in sequence, and dried to serve as a counter electrode.
[0024] (2) Weigh 0.0175 g of solid 4,5-dioxyethylene indole and dissolve it in 5 mL of boron trifluoride ether solution, and configure the reaction of 4,5-dioxyethylene indole monomer concentration of 0.02 mol / L solution. Combine the three electrodes in step (1), put them into the boron trifluoride ether solution containing 4,5-dioxethyleneindole monomer, apply a voltage of 0.5V.vs.SCE, and react for 50s.
[0025] (3) The working electrode in step (2) is taken out, and the poly(4,5-dioxyethylene i...
Embodiment 3
[0027] (1) Put the Ag wire in a hydrochloric acid solution with a concentration of 6mol / L, and after electrolysis at a constant potential of 1.5V for 100s, a red AgCl adhesion layer is formed on the surface as a reference electrode. The ITO glass electrode was wiped clean with alcohol and used as the working electrode. The stainless steel electrode was polished with 1200-mesh sandpaper, rinsed with water, ethanol, and acetone in sequence, and dried to serve as a counter electrode.
[0028] (2) Weigh 0.018 g of solid 4,5-dioxyethylene indole and dissolve it in 5 mL of boron trifluoride ether solution, and configure the reaction in which the monomer concentration of 4,5-dioxyethylene indole is 0.021 mol / L solution. Combine the three electrodes in step (1), put them into the boron trifluoride ether solution containing 4,5-dioxethyleneindole monomer, apply a voltage of 0.5V.vs.SCE, and react for 45s.
[0029] (3) The working electrode in step (2) is taken out, and the poly(4,5-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com