Carbazole fluorescent probe and preparation method and application thereof
A fluorescent probe and carbazole technology, applied in the field of fluorescent probes, can solve problems such as environmental pollution and toxicity, and achieve the effects of high sensitivity, good cell membrane penetration, and simple detection means.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 carbazole fluorescent probe IECBT:
[0049] (1) In a container, mix N-ethylcarbazole-3-carbaldehyde and 2,3-dimethylbenzothiazole iodide at a molar ratio of 1:1 and dissolve in absolute ethanol, and heat to 150 in an oil bath ℃, reflux reaction for 6 hours, take out after cooling;
[0050] (2) After spot plate observation confirmed that the reaction was complete, the mixture was filtered and washed with suction to obtain an orange-red solid product with a yield of 55%.
[0051] Probe for IECBT 1 HNMR, 13 CNMR characterization, the results are as follows:
[0052] 1 HNMR (300MHz, DMSO): δ8.42(d, J=9.0Hz, 3H), 8.22(s, 3H), 8.07(d, J=15.6Hz, 1H), 7.84(m, J=38.4Hz, 4H ),7.56(t,J=15.3Hz,1H),7.37(t,J=14.4Hz,1H),4.55(d,J=6.9Hz,2H),4.37(s,3H),1.39(t,J =13.8Hz,3H)
[0053] 13 CNMR(75MHz,DMSO):δ171.05,149.67,141.30,141.10,139.40,128.26,127.19,126.46,125.95,124.29,123.18,122.81,122.04,121.32,119.72,119.37,115.58,109.20,36.531,35.26,12.94
Embodiment 2
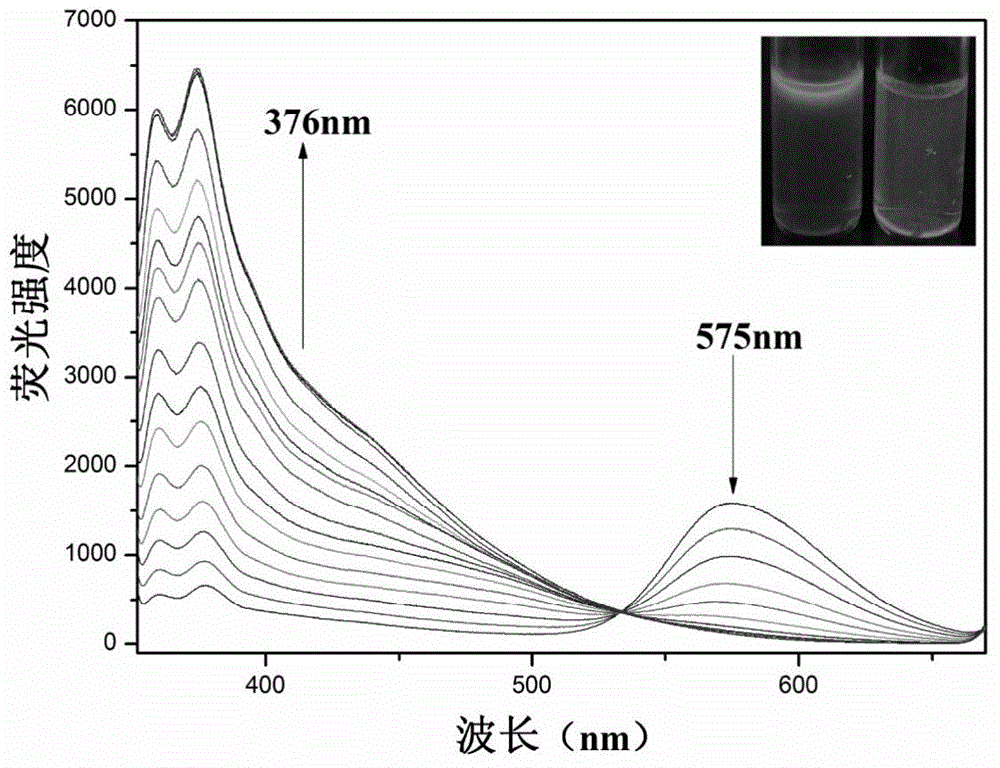
[0054] The CN of embodiment 2 fluorescent probe IECBT - Titration fluorescence plot
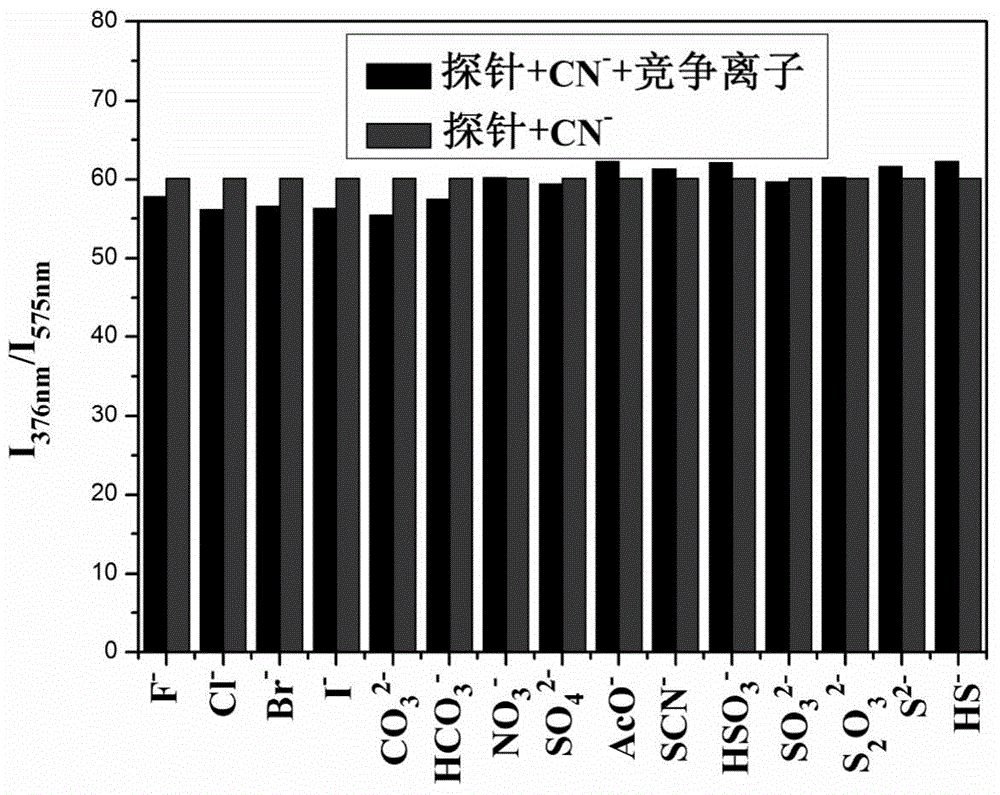
[0055] Add 20 μL fluorescent probe IECBT stock solution to 2 mL DMSO system for CN - Fluorescence titration experiment is detected on a fluorescence spectrophotometer. With the addition of the sample to be tested, the fluorescence intensity at 575nm is gradually weakened, and the emission peaks at 360nm and 376nm are enhanced. Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 10.0nm and 5.0nm respectively, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex 340nm and the maximum fluorescence emission wavelength is: λ em 575nm.
Embodiment 3
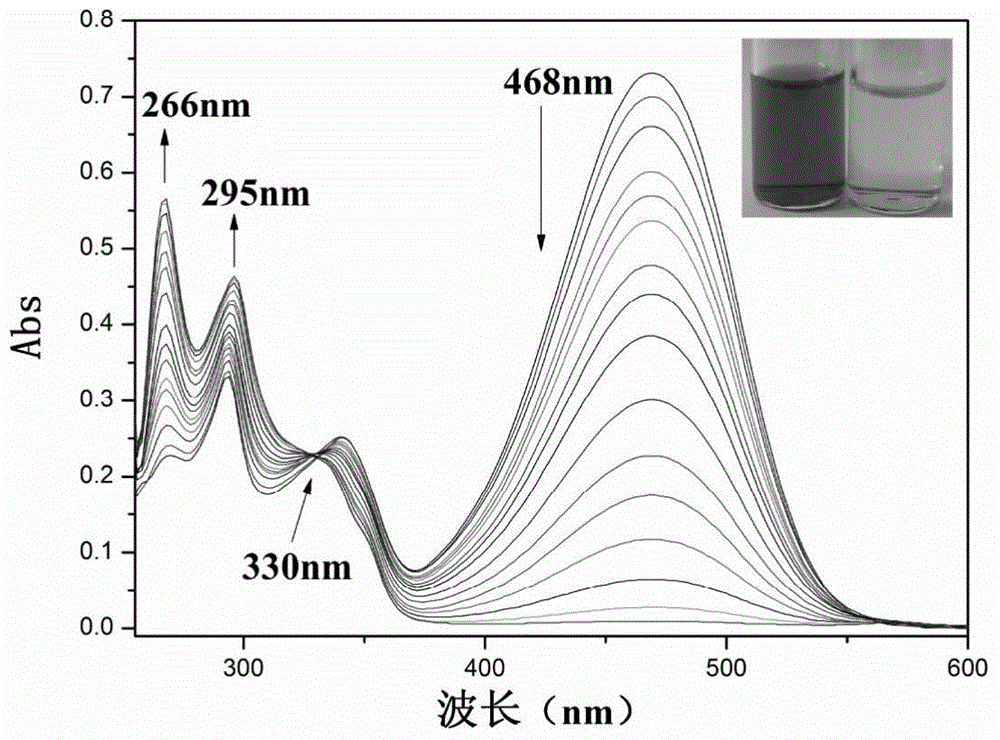
[0056] CN of embodiment 3 fluorescent probe IECBT - Titration UV diagram
[0057] Add 30 μL fluorescent probe IECBT stock solution to 2 mL DMSO system for CN - The ultraviolet titration experiment is detected on the ultraviolet spectrophotometer. With the addition of the sample to be tested, the ultraviolet absorption intensity at 468nm gradually weakens, and the ultraviolet absorption intensity at 266nm and 295nm gradually increases.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com