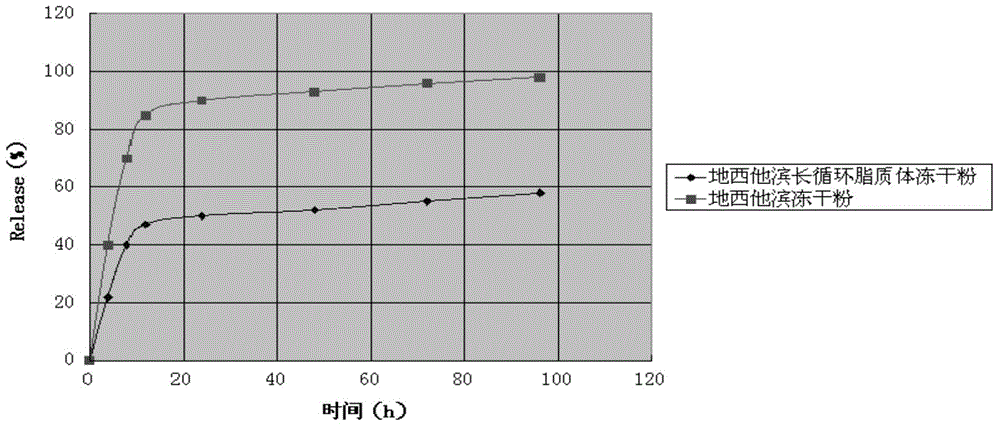
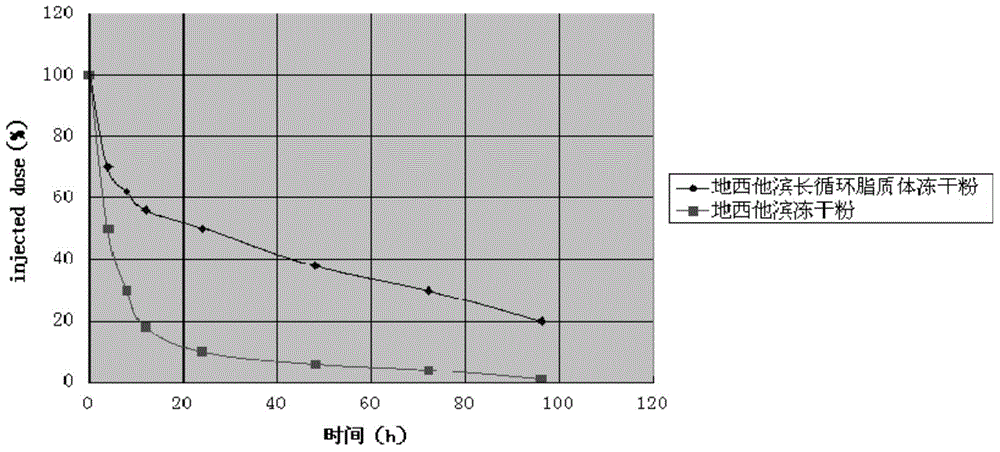
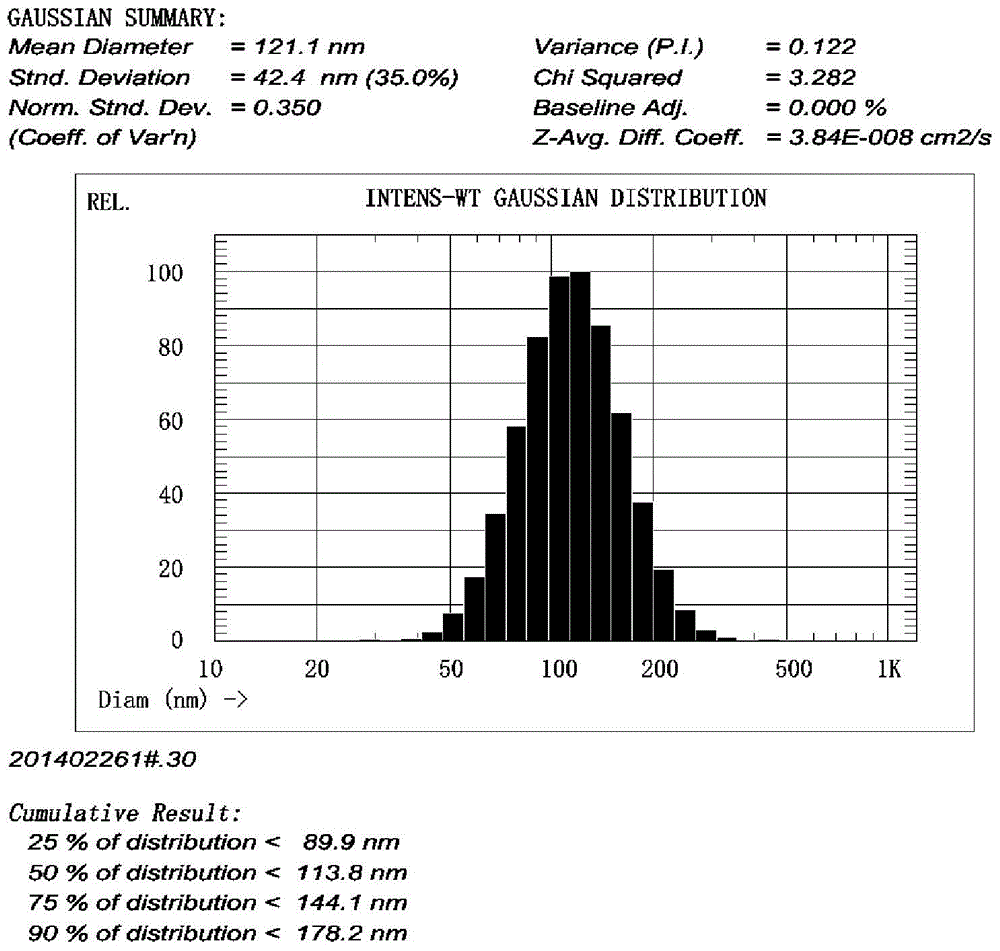
Decitabine long-circulating liposome lyophilized preparation and preparation method thereof
A long-circulation liposome and liposome freeze-drying technology, which can be used in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of reducing the rapid phagocytosis of the reticuloendothelial system Slow down plastid clearance rate, prolonged drug action time and other problems, to achieve the effect of good appearance, small integrity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] 1、地西他滨长循环脂质体制剂的制备实施例
[0046] (1)不同配比磷脂制备的地西他滨长循环脂质体制剂
[0047] 例1.1处方:
[0048]
[0049] Preparation:
[0050] (1)秤取处方量的地西他滨、PG、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0051] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0052] (3)再将其用高压均质机挤压,压力1000bar,均质4遍,便可得地西他滨长循环脂质体。
[0053] 例1.2处方:
[0054]
[0055] Preparation:
[0056] (1)秤取处方量的地西他滨、PS、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0057] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0058] (3)再将其用高压均质机挤压,压力800bar,均质8遍,便可得地西他滨长循环脂质体。
[0059] 例1.3处方:
[0060]
[0061] Preparation:
[0062] (1)秤取处方量的地西他滨、HSPC、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0063] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0064] (3)再将其用高压均质机挤压,压力1000bar,均质5遍,便可得地西他滨长循环脂质体。
[0065] (2)不同配比胆固醇制备的地西他滨长循环脂质体制剂
[0066] 例2.1处方:
[0067]
[0068]
[0069] Preparation:
[0070] (1)秤取处方量的地西他滨、HSPC、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶...
example 22
[0073] 例2.2处方
[0074]
[0075] Preparation:
[0076] (1)秤取处方量的地西他滨、HSPC、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0077] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0078] (3)再将其用高压均质机挤压,压力1000bar,均质3遍,便可得地西他滨长循环脂质体。
example 23
[0079] 例2.3处方
[0080]
[0081] Preparation:
[0082] (1)秤取处方量的地西他滨、HSPC、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0083] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0084] (3)再将其用高压均质机挤压,压力1000bar,均质4遍,便可得地西他滨长循环脂质体。
[0085] (3)不同PH值磷酸盐缓冲液制备的地西他滨长循环脂质体制剂
[0086] 例3.1处方:
[0087]
[0088]
[0089] Preparation:
[0090] (1)秤取处方量的地西他滨、PG、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0091] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0092] (3)再将其用高压均质机挤压,压力700bar,均质8遍,便可得地西他滨长循环脂质体。
[0093] 例3.2处方:
[0094]
[0095] Preparation:
[0096] (1)秤取处方量的地西他滨、PG、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质在器壁上形成薄膜。
[0097] (2)加入磷酸盐缓冲液,进行振摇,则可形大多层脂质体,其粒径范围约1-5um。
[0098] (3)再将其用高压均质机挤压,压力1000bar,均质3遍,便可得地西他滨长循环脂质体。
[0099] 例3.3处方:
[0100]
[0101] Preparation:
[0102] (1)秤取处方量的地西他滨、PG、PEG-DSPE、胆固醇,将其溶解于处方量氯仿和甲醇的混合溶剂中,然后旋转蒸发仪在30℃以下减压旋转下除去有机溶剂,使脂质体在器壁上形成薄膜。
[0103] (2)加入磷酸盐缓冲液,进行...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com