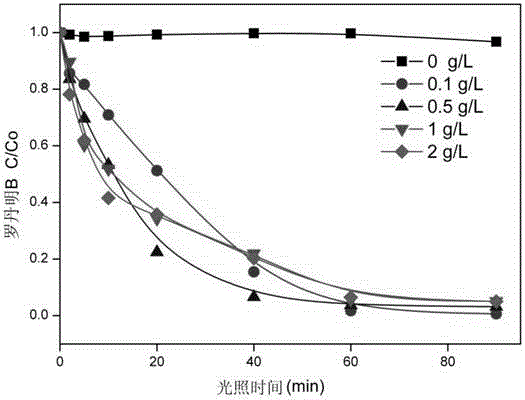
ZnFe2O4/BiOBr magnetic photocatalyst and preparation method thereof
A photocatalyst, znfe2o4 technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of secondary pollution, separation and recovery, human health and environmental hazards, and achieve low cost, Easy-to-control, actionable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
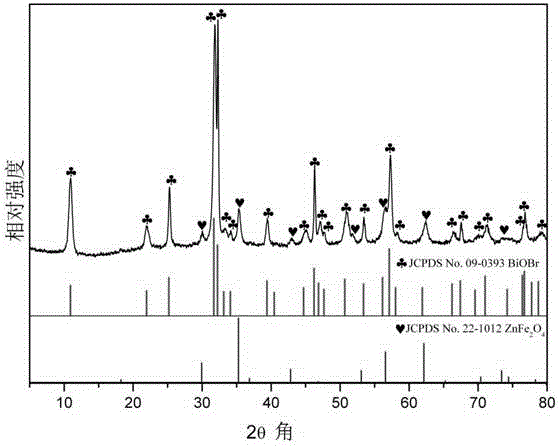
[0034] A ZnFe 2 o 4 The preparation method of / BiOBr magnetic photocatalyst specifically comprises the following steps:
[0035] a. ZnCl 2 and FeCl 3 ·6H 2 O is dissolved in ethylene glycol solution with a molar ratio of 1:0.5-3, and stirred evenly to obtain solution A;
[0036] b. Add NaAc and polyethylene glycol to the solution A, stir evenly to obtain solution B;
[0037] c. Transfer the solution B to a reaction kettle, heat and react to obtain solution C and precipitate D;
[0038] d. Separate the solution C and the precipitate D, wash the precipitate D with ethanol, and dry it, the precipitate D is ZnFe 2 o 4 ;
[0039] e. Bi(NO 3 ) 3 ·5H 2 O and the precipitate D are dissolved in the acetic acid solution with a molar ratio of 1:0.6-1.2, and stirred evenly to obtain a solution E;
[0040] f. KBr is added to the solution E at a molar ratio twice that of the precipitate D, stirred evenly, and aged to obtain solution F and precipitate G;
[0041] g. Separate the ...
Embodiment 1
[0050] 2.5mmol of ZnCl 2 and 5 mmol FeCl 3 ·6H 2 O was dissolved in 40mL of ethylene glycol solution and stirred evenly to obtain solution A;
[0051] Weigh 3.6g of NaAc and 1.0g of polyethylene glycol 20000, pour them into the above solution A, and stir evenly, then mechanically stir the solution for 30min to obtain solution B;
[0052] Transfer the solution B to a 50mL reaction kettle, react at 200°C for 8 hours, separate the solution C and the precipitate D, wash the precipitate D with absolute ethanol 3 times, and dry it at 60°C for 6h, the obtained precipitate D is ZnFe 2 o 4 ,spare;
[0053] Weigh 2mmolZnFe 2 o 4 and 2mmol Bi(NO 3 ) 3 5H2O was ultrasonically dispersed into 100mL aqueous solution containing 5mL acetic acid, and stirred evenly at room temperature to obtain solution E;
[0054] Weigh 2mmol KBr, pour it into the above solution E, and stir evenly, then mechanically stir the solution for 60min, and age for 3h to obtain solution F and precipitate G; ...
Embodiment 2
[0058] 2.5mmol of ZnCl 2 and 1.25 mmol FeCl 3 ·6H 2 O was dissolved in 40mL of ethylene glycol solution and stirred evenly to obtain solution A;
[0059] Weigh 3.6g of NaAc and 1.0g of polyethylene glycol 20000, pour them into the above solution A, and stir evenly, then mechanically stir the solution for 30min to obtain solution B;
[0060] Transfer the solution B to a 50mL reaction kettle, react at 200°C for 8 hours, separate the solution C and the precipitate D, wash the precipitate D with absolute ethanol 3 times, and dry it at 60°C for 6h, the obtained precipitate D is ZnFe 2 o 4 ,spare;
[0061] Weigh 2mmolZnFe 2 o 4 and 2mmol Bi(NO 3 ) 3 ·5H 2 O was ultrasonically dispersed into 100 mL of aqueous solution containing 5 mL of acetic acid, and stirred evenly at room temperature to obtain solution E;
[0062] Weigh 2mmol KBr, pour it into the above solution E, and stir evenly, then mechanically stir the solution for 60min, and age for 3h to obtain solution F and p...
PUM
| Property | Measurement | Unit |
|---|---|---|
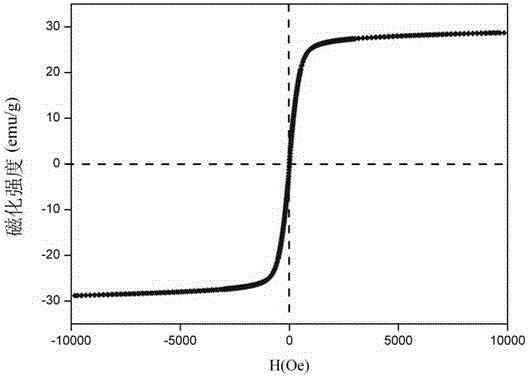
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com