4-fatty sulfoamide-2-nitrobenzyl bromide compounds and synthetic method thereof
A technology of nitrobenzyl bromide and sulfonamide group, applied in the field of surfactant synthesis chemistry, can solve problems such as difficulty in chlorosulfonation, and achieve the effects of mild conditions, simple synthesis method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Synthesis of intermediate 4-sulfonyl chloride-2-nitrotoluene
[0037] Add 4.64g (0.04mol) of chlorosulfonic acid into the three-necked flask, dropwise add 1.37g (0.01mol) o-nitrotoluene solution below 0°C, and keep stirring below 0°C for 60min. The temperature was programmed to rise to 43°C, and the temperature was maintained for 18 hours. After the reaction, the reaction solution was slowly added dropwise to ice water, a large amount of acid mist was generated, and the lower layer was left standing to form a milky white oily liquid, and then the lower layer was extracted with n-butanol to obtain the n-butanol layer, and the n-butanol was removed. Separated by eluent, 2.06 g of 4-sulfonyl chloride-2-nitrotoluene was obtained with a yield of 87.6%.
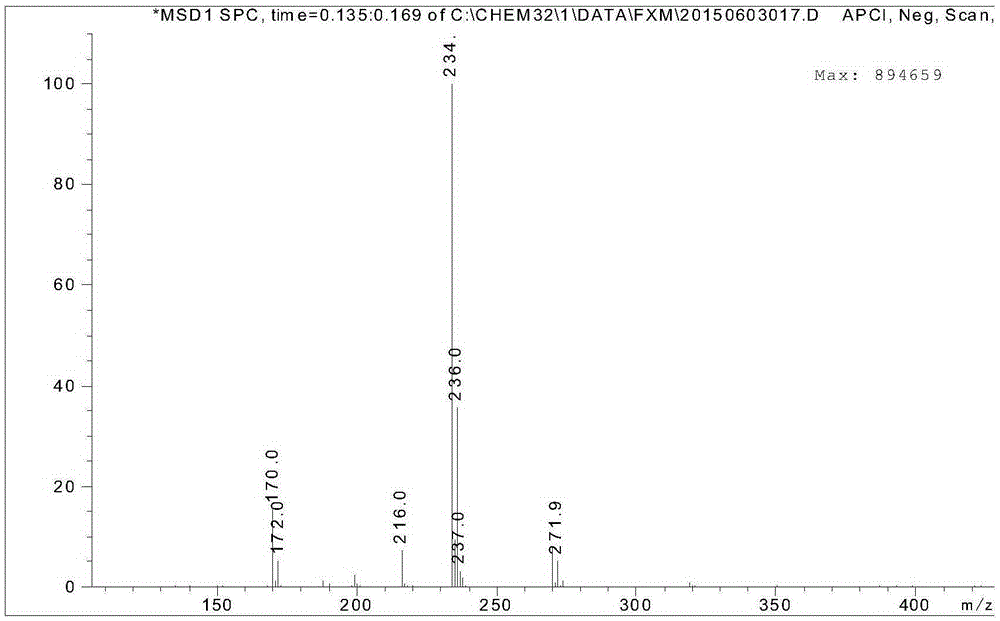
[0038] figure 1 It is the mass spectrum of 4-sulfonyl chloride-2-nitrotoluene, where m / z234.0 is the target product [M-H] - Peak, 270.0 is the target product [M+Cl] - peak;
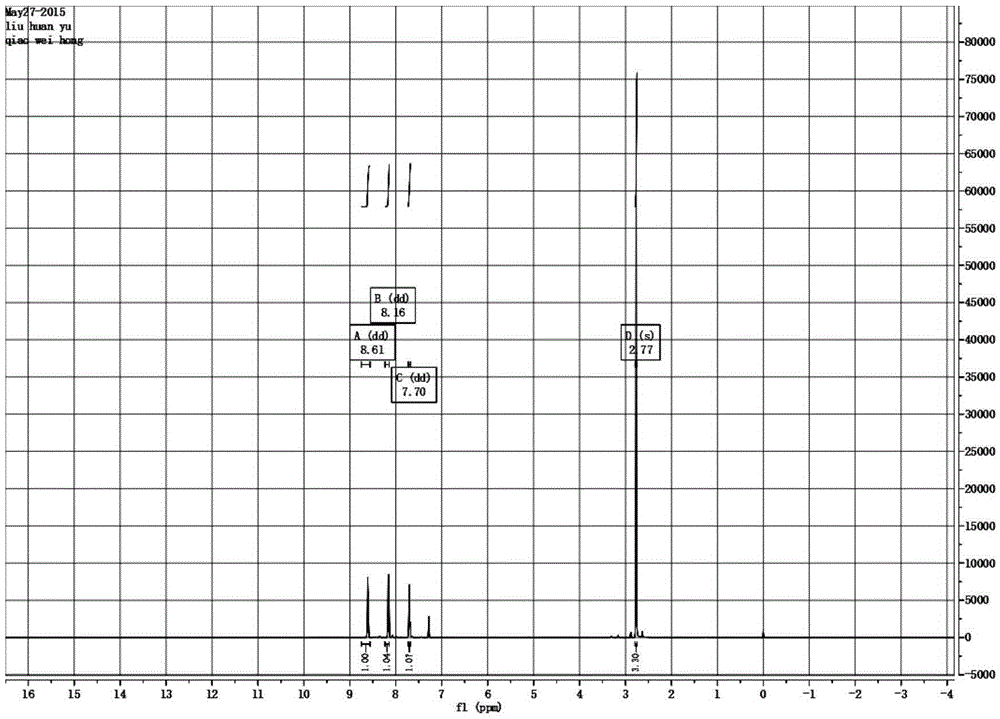
[0039] figure 2It is the H NMR spectru...
Embodiment 2
[0049] (1) Synthesis of intermediate 4-sulfonyl chloride-2-nitrotoluene
[0050] Add 5.80g (0.05mol) of chlorosulfonic acid into the reaction flask, add dropwise 1.37g (0.01mol) o-nitrotoluene solution below 0°C, and keep stirring at 0°C for 50min. The temperature was raised to 38°C, and the reaction temperature was maintained for 17 hours. After the reaction, the reaction solution was slowly added dropwise to ice water, a large amount of acid mist was generated, and the lower layer was left standing to form a milky white oily liquid, and then the lower layer was extracted with ethyl acetate to obtain the ethyl acetate layer, and the ethyl acetate was removed. Separated by eluent, 1.97 g of 4-sulfonyl chloride-2-nitrotoluene was obtained, with a yield of 83.8%;
[0051] (2) Synthesis of intermediate 4-(N,N-di-n-decyl)-sulfonamido-2-nitrotoluene
[0052] Dissolve 1.18g (0.005mol) of the intermediate 4-sulfonyl chloride-2-nitrotoluene in 18mL of chloroform, add 1.48g (0.005mol...
Embodiment 3
[0056] (1) Synthesis of intermediate 4-sulfonyl chloride-2-nitrotoluene
[0057] With embodiment 1 (1).
[0058] (2) Synthesis of intermediate 4-(N-dodecyl)-sulfonamido-2-nitrotoluene
[0059] Dissolve 1.18g (0.005mol) of the intermediate 4-sulfonyl chloride-2-nitrotoluene in 18mL of carbon tetrachloride, add 0.92g (0.005mol) of n-dodecylamine dropwise, raise the temperature to 51°C, and continuously add Adjust the pH to 7-11 with sodium hydroxide, and react for 12 hours; after the reaction, concentrate the reaction solution under reduced pressure, filter it with suction, dry it in vacuum, and separate it with an eluent column to obtain the product 4-(N-dodecyl) -sulfonamido-2-nitrotoluene.
[0060] (3) Synthesis of product 4-(N-dodecyl)-sulfonamido-2-nitrobenzyl bromide
[0061] Dissolve 1.92g (0.005mol) of 4-(N-dodecyl)-sulfonamido-2-nitrotoluene in 20mL of chloroform, add 0.24g of azobisisoheptanonitrile, and heat up to 70-85°C , add sodium bromide 0.51g (0.005mol) in b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com