Catalyst for preparing trichloroethylene from 1,1,1,2-tetrafluoroethane
A technology of trifluoroethylene and tetrafluoroethane, used in physical/chemical process catalysts, dehydrohalogenation preparation, organic chemistry, etc., can solve the problems of low catalytic activity and fast deactivation of catalysts, and achieve high catalytic activity and catalyst Long life, short life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 1 mol of alumina in a polytetrafluoroethylene reaction bottle, add water and stir to obtain a slurry, add 90 g of hydrofluoric acid with a mass concentration of 40% dropwise, filter, wash the filter cake until neutral, and dry it in an oven at 80 ° C. Calcined at 430°C for 4h in a muffle furnace to obtain Al 2 o 2.1 f 1.8 , the Al 2 o 2.1 f 1.8 Put into the flask, impregnate ferric nitrate, ferric nitrate and Al 2 o 2.1 f 1.8 The mass ratio is 0.01:1, dried in an oven at 80°C, and calcined in a muffle furnace at 430°C for 4h to make a catalyst 0.01Fe / Al 2 o 2.1 f 1.8 . Through evaluation, the conversion rate of HFC-134a is 63.2%, and the selectivity of trifluoroethylene reaches 98.9%.
Embodiment 2
[0017] Add 1 mol of alumina in a polytetrafluoroethylene reaction bottle, add water and stir to obtain a slurry, add 120 g of hydrofluoric acid with a mass concentration of 40% dropwise, filter, wash the filter cake until neutral, and dry it in an oven at 90 ° C. Calcined at 450°C for 3h in a muffle furnace to obtain Al 2 o 1.8 f 2.4 , the Al 2 o 1.8 f 2.4 Put into the flask, impregnate ferric nitrate, ferric nitrate and Al 2 o 1.8 f 2.4 The mass ratio of 0.05:1, dried in an oven at 90 ° C, roasted in a muffle furnace at 450 ° C for 3 hours, made into a catalyst, made into a catalyst 0.05Fe / Al 2 o 1.8 f 2.4 . Through evaluation, the conversion rate of HFC-134a is 67.4%, and the selectivity of trifluoroethylene reaches 99.2%.
Embodiment 3
[0019] Add 1 mol of alumina in a polytetrafluoroethylene reaction bottle, add water and stir to obtain a slurry, add 180 g of hydrofluoric acid with a mass concentration of 40% drop by drop, filter, wash the filter cake until neutral, and dry it in an oven at 100 ° C. Calcined at 470°C for 3h in a muffle furnace to obtain Al 2 o 1.2 f 3.6 , the Al 2 o 1.2 f 3.6 Put into the flask, impregnate ferric nitrate, ferric nitrate and Al 2 o 1.2 f 3.6 The mass ratio is 0.15:1, dried in an oven at 100°C, and roasted in a muffle furnace at 470°C for 3 hours to make a catalyst 0.15Fe / Al 2 o 1.2 f 3.6 . Through evaluation, the conversion rate of HFC-134a is 68.7%, and the selectivity of trifluoroethylene reaches 99.0%.
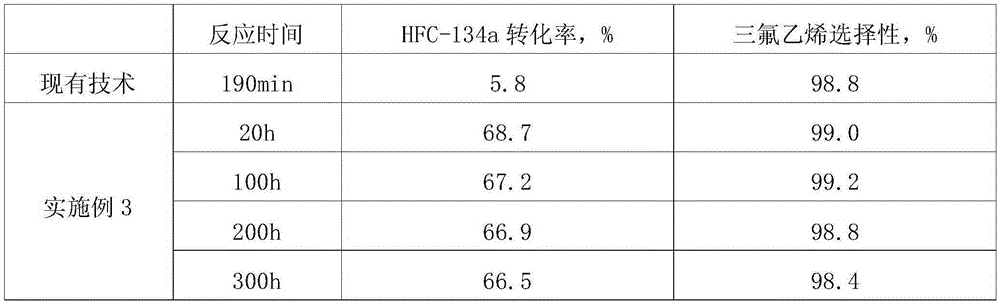
[0020] The catalyst of Example 3 was subjected to a life test, and the evaluation results are shown in Table 1.
[0021] Table 1 Life Test
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com