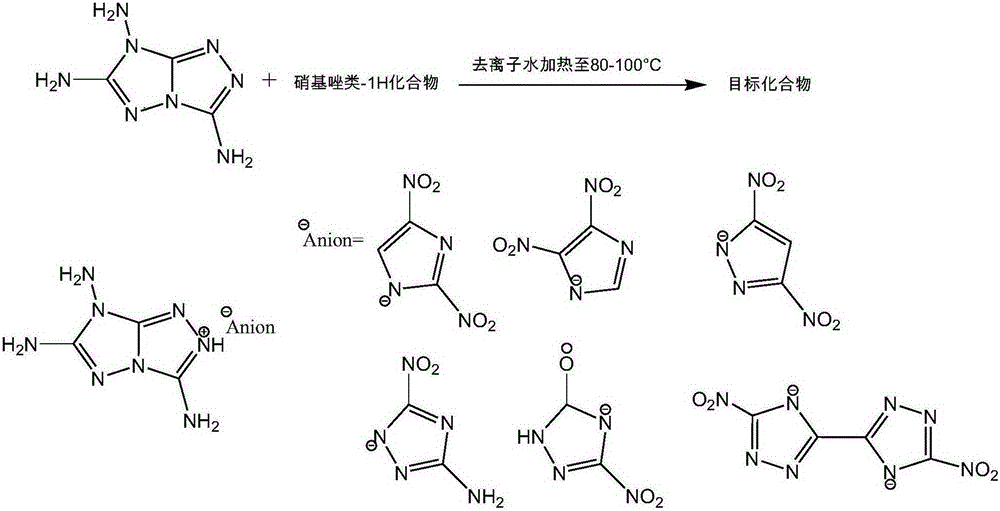
3,6,7-triamino-1,2,4-triazolocycle ionic type nitroazole compound and preparation method thereof
A technology for nitroazoles and cyclic compounds, applied in the direction of organic chemistry and the like, can solve the problems of low mechanical sensitivity of detonation performance, and achieve the effects of non-toxic by-products, mild conditions and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The structure of 3,6,7-triamino-1,2,4-triazolocyclo-2,4-dinitroimidazole is as follows:
[0034]
[0035] Its preparation method is as follows:
[0036] Add 158 mg (1 mmol) of 2,4-dinitroimidazole-1H to 10 mL (0.5 mol) of aqueous solution, and add 154 mg of 3,6,7-triamino-1,2,4-triazolocyclic compound under stirring ( 1mmol), heated to 80°C, the solution turned into a light yellow transparent solution, continued to stir, reacted for 1h and then stood still. After the temperature dropped to room temperature, yellow crystals were precipitated, filtered out and washed with water, and dried to obtain the target compound 3,6,7-tri Amino-1,2,4-triazolocyclo-2,4-dinitroimidazole 280 mg, yield 89.7%.
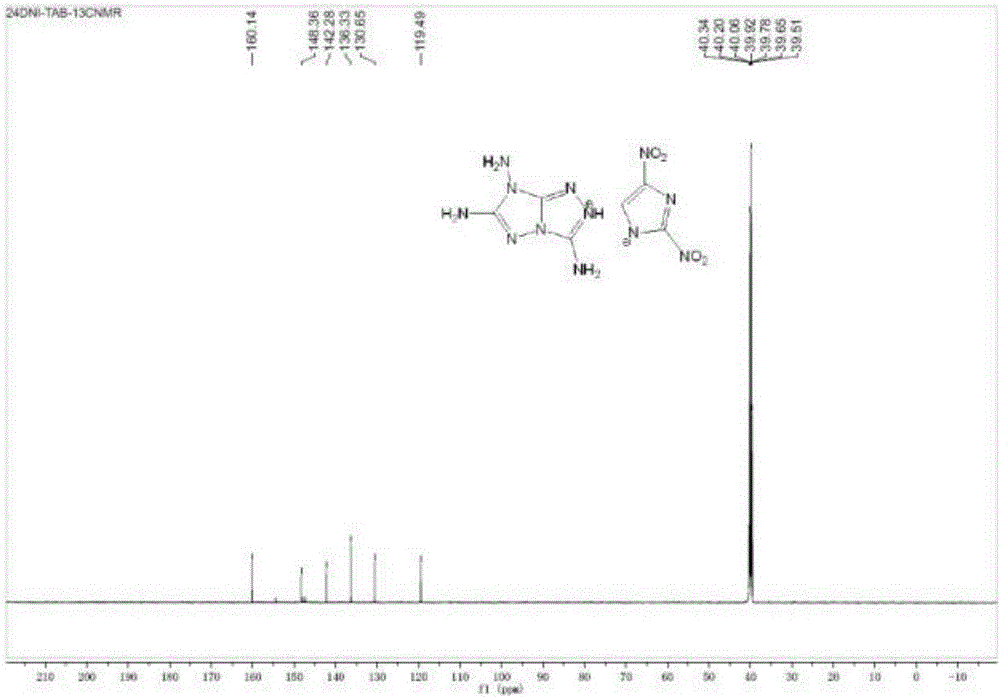
[0037] 3,6,7-triaminotriazolocyclo-2,4-dinitroimidazole 1 H NMR (DMSO, δ, ppm): 8.18(2H), 7.30(1H), 7.24(2H), 5.78(2H); 13 C NMR such as figure 2 Shown (DMSO, δ, ppm): 160.61, 156.83, 147.91, 141.61, 98.83; Infrared (KBr, cm -1 ): 3420(s), 3357(m), 3255(s), 1679(s), 1650...
Embodiment 2
[0039] The conditions were the same as in Example 1, but the reaction temperature was changed to 90°C and the reaction time was changed to 0.5h to obtain 295 mg of the target compound 3,6,7-triaminotriazolocyclo-2,4-dinitroimidazole, with a yield of 94.5 %.
Embodiment 3
[0041] The structure of 3,6,7-triamino-1,2,4-triazolocyclo-4,5-dinitroimidazole is as follows:
[0042]
[0043] Its preparation method is as follows:
[0044]Add 158 mg (1 mmol) of 4,5-dinitroimidazole-1H to 10 mL (0.5 mol) of aqueous solution, and add 154 mg of 3,6,7-triamino-1,2,4-triazolocyclic compound under stirring ( 1mmol), heated to 85°C, the solution turned into a light yellow transparent solution, continued to stir, reacted for 0.5h and then stood still. After the temperature dropped to room temperature, yellow crystals were precipitated, filtered out, washed with water, and dried to obtain the target compound 3,6,7- Triaminotriazolocyclo-4,5-dinitroimidazole 290 mg, yield 92.9%.
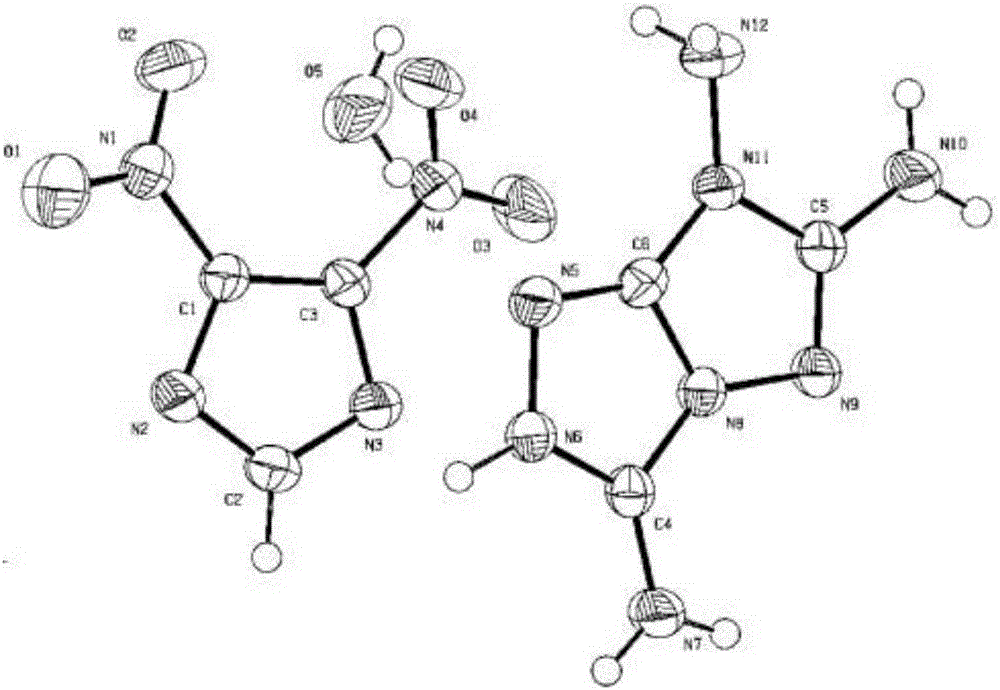
[0045] After recrystallization, a single crystal of 3,6,7-triaminotriazolocyclo-4,5-dinitroimidazole monohydrate was obtained, the crystal structure of which is image 3 As shown, the space group is P-1, the unit cell parameters are α=79.688(5)°, β=74.117(6)°, γ=80.828(5)°, and the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com