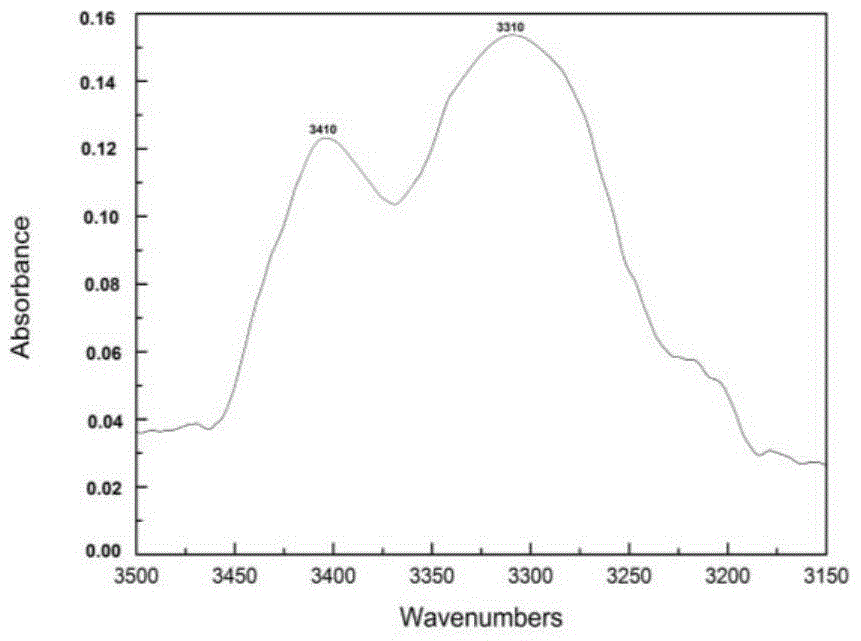
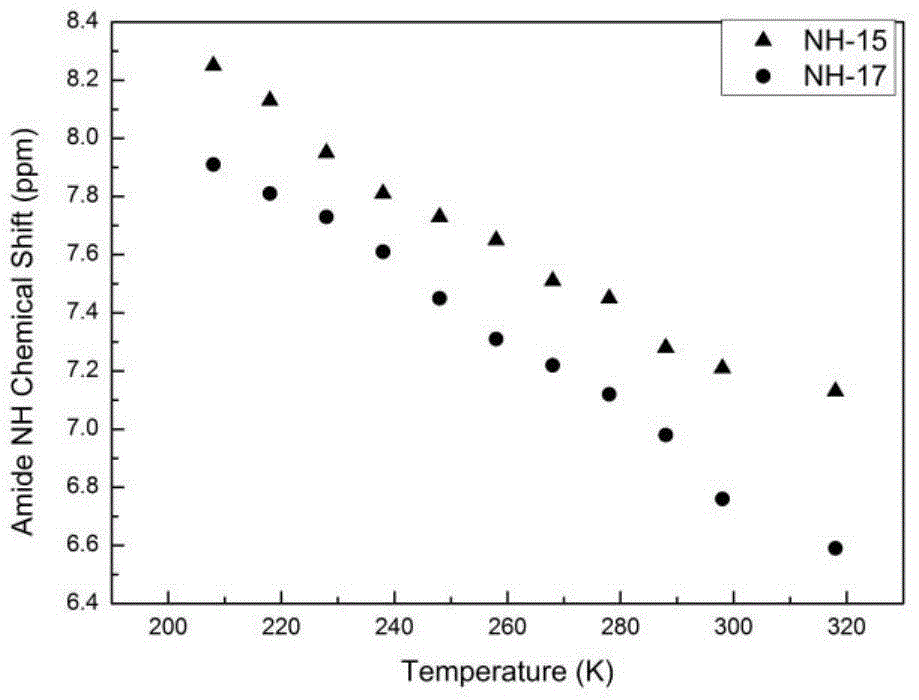
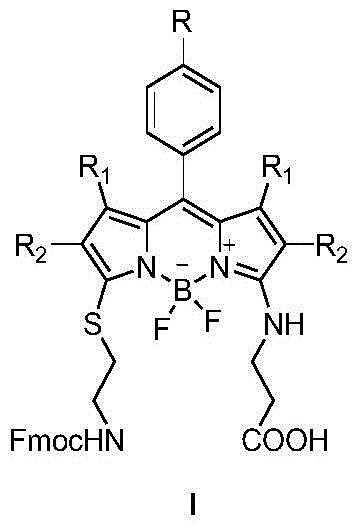
A bodipy-based fluorescent amino acid and its synthesis method and application
A synthetic method and amino acid technology, applied in the field of medical bioengineering, can solve problems affecting the biological activity of polypeptides, time-consuming, and labor-intensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] a. Preparation of Intermediate I-1:
[0078] Take a dry 500ml two-neck flask, replace the bottle with nitrogen, add pyrrole (104ml, 1.5mol), benzaldehyde (6mL, 60mmol) and catalyst trifluoroacetic acid (0.67mL, 6mmol), stir at room temperature for 1h, and add a concentration of 1M Quenched with NaOH solution (100 mL), extracted with ethyl acetate (200 mL×3), dried over anhydrous magnesium sulfate, filtered, concentrated, and recrystallized from ethanol to obtain 10.5 g of a brown solid with a yield of 80.7%.
[0079] b. Preparation of Intermediate I-2:
[0080] Intermediate I-1 (5g, 22mmol) was dissolved in 200mL of anhydrous tetrahydrofuran, replaced with a nitrogen system, and a tetrahydrofuran solution (60mL) of chlorosuccinimide (6.5g, 48.4mmol) was added at -78°C, Keep stirring at -78°C for 2 hours, then stir at room temperature for 3 hours. After the reaction, add water (300 mL), extract with dichloromethane (200 mL×3), dry over anhydrous magnesium sulfate, filte...
Embodiment 2
[0105] a. Preparation of Intermediate I-1:
[0106] Take a dry 500ml two-neck flask, replace the bottle with nitrogen, add pyrrole (83.2ml, 1.2mol), benzaldehyde (6mL, 60mmol) and trifluoroacetic acid (1.34mL, 12mmol), stir at room temperature for 2h, add 1M NaOH solution (100 mL), extracted with ethyl acetate (200 mL×3 times), dried over anhydrous magnesium sulfate, filtered, concentrated, and recrystallized from ethanol to obtain intermediate I-1 as a brown solid.
[0107] b. Preparation of Intermediate I-2:
[0108] Intermediate I-1 (5g, 22mmol) was dissolved in 200mL of anhydrous tetrahydrofuran, replaced with a nitrogen system, a tetrahydrofuran solution (60mL) of chlorosuccinimide (8.86g, 66mmol) was added at -78°C, and kept Stir at -78°C for 3 h, then at room temperature for 4 h. After the reaction, add water (300 mL), extract with dichloromethane (200 mL x 3 times), dry over anhydrous magnesium sulfate, filter, and concentrate. Dissolve the obtained product in dichlo...
Embodiment 3
[0129]a. Preparation of Intermediate I-1:
[0130] Take a dry 500ml two-neck flask, replace the bottle with nitrogen, add pyrrole (93.6ml, 1.35mol), benzaldehyde (6mL, 60mmol) and trifluoroacetic acid (1.01mL, 9mmol), stir at room temperature for 1.5h, add 1M NaOH The solution (100 mL) was quenched, extracted with ethyl acetate (200 mL×3 times), dried over anhydrous magnesium sulfate, filtered, concentrated, and recrystallized from ethanol to obtain intermediate I-1 as a brown solid.
[0131] b. Preparation of Intermediate I-2:
[0132] Intermediate I-1 (5g, 22mmol) was dissolved in 300mL of anhydrous tetrahydrofuran, replaced with nitrogen system, and a tetrahydrofuran solution (100mL) of chlorosuccinimide (7.39g, 55mmol) was added at -78°C, and kept Stir at -78°C for 2.5 h, then at room temperature for 3.5 h. After the reaction, add water (300 mL), extract with dichloromethane (200 mL x 3 times), dry over anhydrous magnesium sulfate, filter, and concentrate. Dissolve the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com