A kind of preparation method of calcium fluoride microsphere
A technology of calcium fluoride and microspheres, applied in the direction of calcium/strontium/barium fluoride, calcium/strontium/barium halide, etc., can solve the problems of high production cost and harsh reaction conditions, and achieve convenient operation and mild reaction conditions , The effect of simple preparation process equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
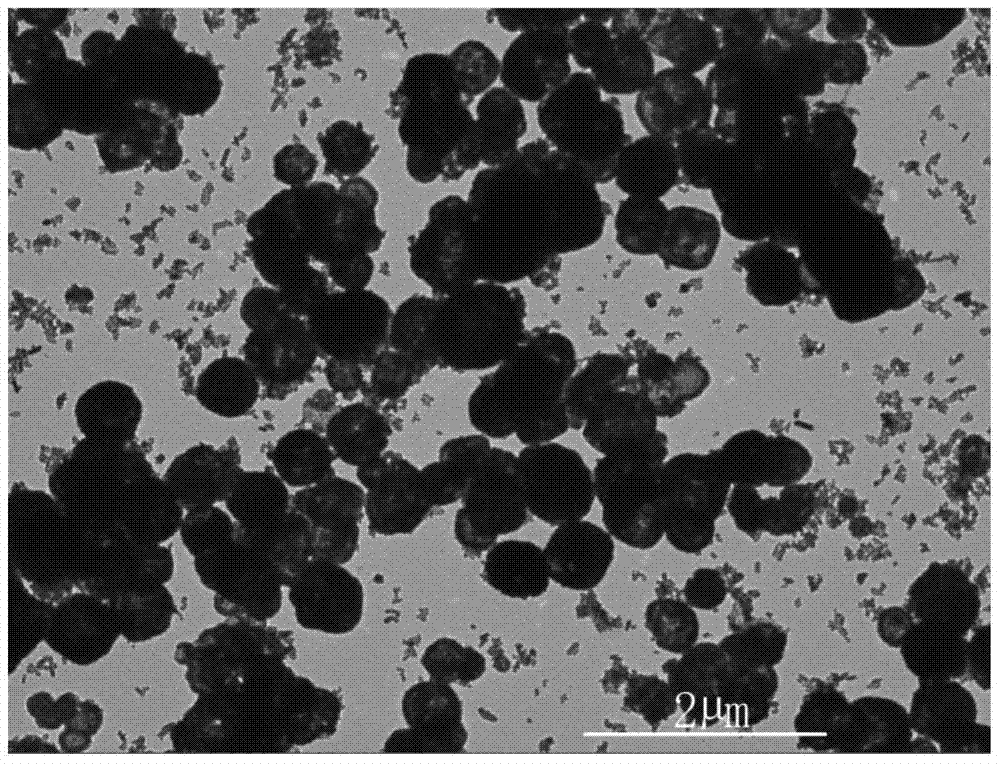
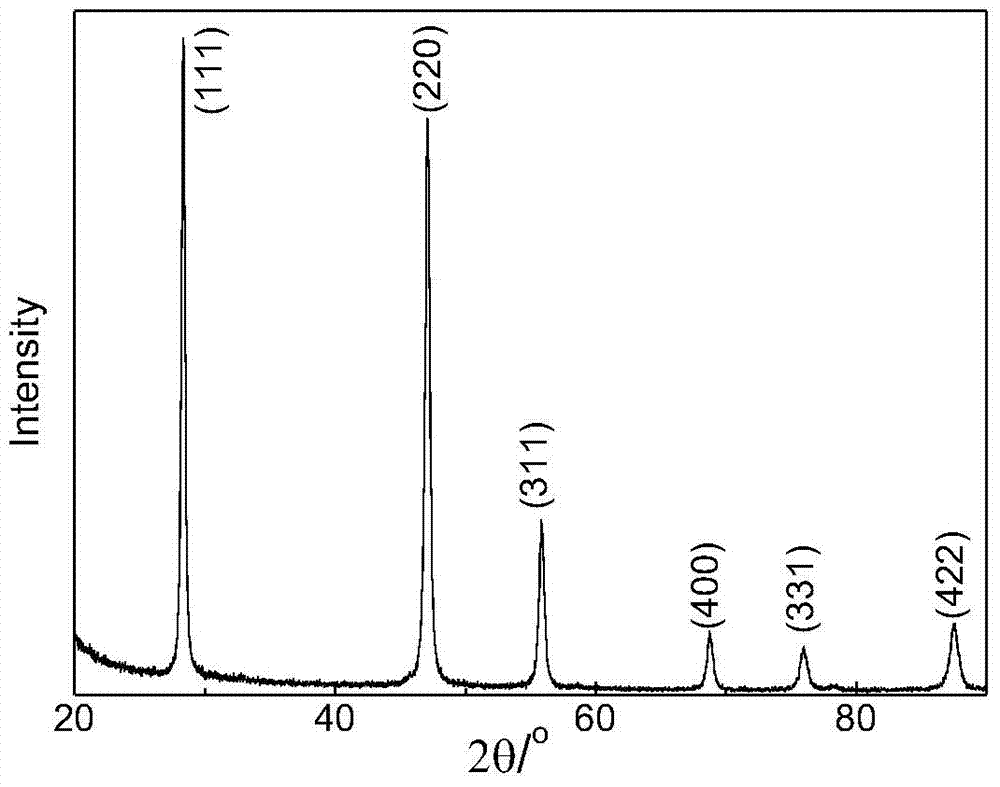
[0030] 1 g of commercially available octacalcium phosphate (Ca 8 (HPO 4 ) 2 (PO 4 )·5H 2 O) powder, put into 500mL distilled water, stir with magnetic stirrer, with the phosphoric acid (H of 0.01mol / L NaOH solution and 0.01mol / L 3 PO 4 ) to carry out acid-base titration to keep the pH value of the solution at 5.2, the temperature is 20°C, stir with a magnetic stirrer for 1 hour, and add 0.5mol / L of NH 4 F solution 100mL, continue to stir for 24h. The suspension was filtered with distilled water, and when the pH value of the filtered solution was neutral, it was filtered twice with absolute ethanol, and dried in an environment of 40°C to obtain calcium fluoride (CaF 2 )Microspheres. figure 1 and figure 2 calcium fluoride (CaF 2 ) transmission electron microscope picture and XRD pattern, by figure 1 It can be seen that the product is a hollow microsphere with a diameter of 200nm to 800nm, the size of the microsphere is relatively uniform, the shape is consistent, and ...
Embodiment 2
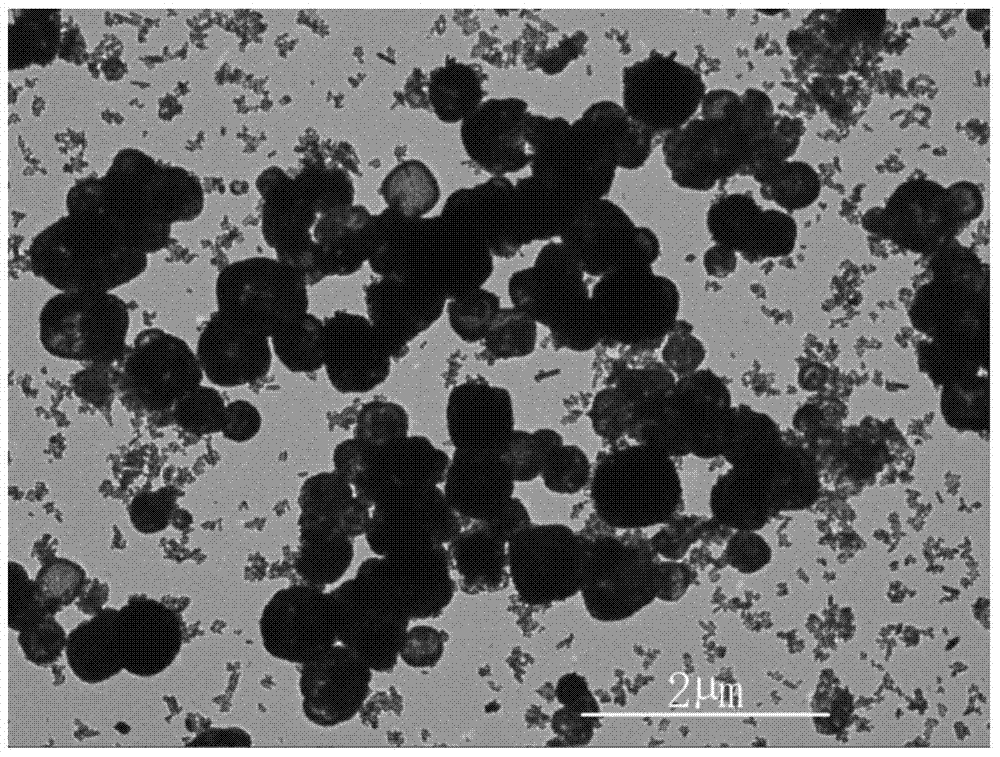
[0032] 1 g of commercially available hydroxyapatite (Ca 10 (PO 4 ) 6 (OH) 2) powder, put into 500mL distilled water, stir with a magnetic stirrer, use 0.01mol / L NaOH solution and 0.01mol / L phosphoric acid (H 3 PO 4 ) to carry out acid-base titration to keep the pH value of the solution at 5.5, the temperature is 20°C, stir with a magnetic stirrer for 1 hour, and add 0.5mol / L of NH 4 F solution 100mL, continue to stir for 24h. The suspension was filtered with distilled water, and when the pH value of the filtered solution was neutral, it was filtered twice with absolute ethanol, and dried in an environment of 40°C to obtain calcium fluoride (CaF 2 )Microspheres. image 3 and Figure 4 calcium fluoride (CaF 2 ) transmission electron microscope picture and XRD pattern, image 3 It can be seen that the product is a hollow microsphere with a diameter of 200nm to 800nm, the size of the microsphere is relatively uniform, the shape is consistent, and it is approximately spher...
Embodiment 3
[0034] Commercially available 1 g β-tricalcium phosphate (Ca 3 (PO 4 ) 2 ) powder, put into 500mL distilled water, stir with a magnetic stirrer, use 0.01mol / L NaOH solution and 0.01mol / L phosphoric acid (H 3 PO 4 ) to carry out acid-base titration to keep the pH value of the solution at 6.0, the temperature is 20°C, stir with a magnetic stirrer for 1 hour, and add 0.5mol / L of NH 4 F solution 100mL, continue to stir for 24h. The suspension was filtered with distilled water, and when the pH value of the filtered solution was neutral, it was filtered twice with absolute ethanol, and dried in an environment of 40°C to obtain calcium fluoride (CaF 2 )Microspheres. Figure 5 and Figure 6 calcium fluoride (CaF 2 ) transmission electron microscope picture and XRD pattern, Figure 5 It can be seen that the product is a hollow microsphere with a diameter of 200nm to 800nm, the size of the microsphere is relatively uniform, the shape is consistent, and it is approximately spheri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com