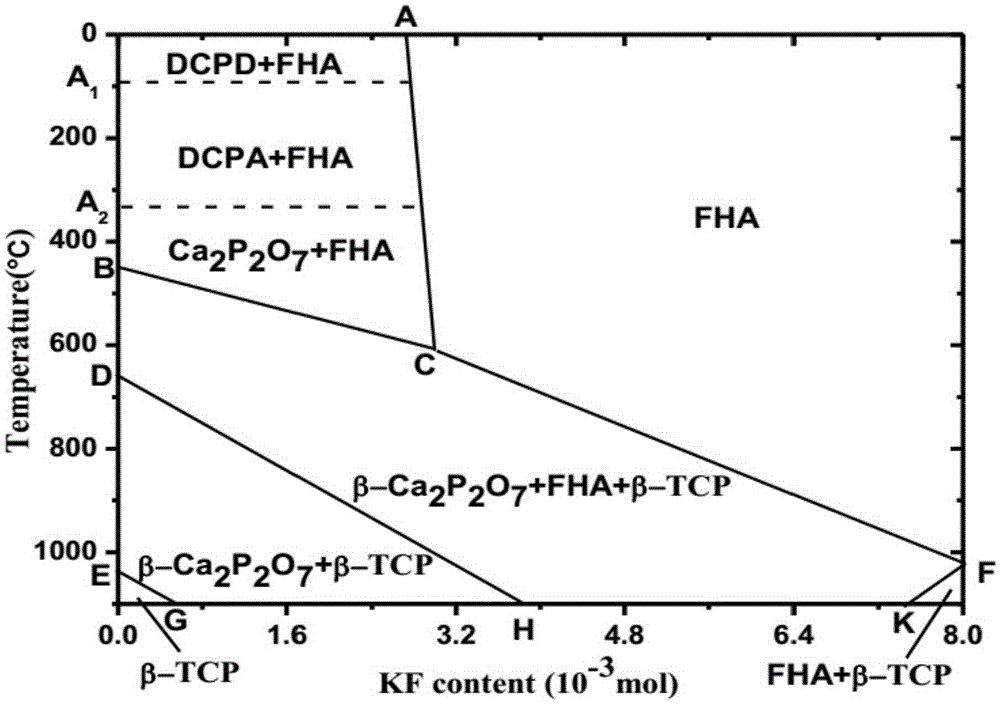
FHA/beta-TCP/beta-Ca2P2O7 three-phase ceramic preparation method
A technology for preparation of -ca2p2o7, ceramics, applied in the field of biomedical materials, can solve the problem that the harmful biological effects of alumina nanoparticles cannot be completely removed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
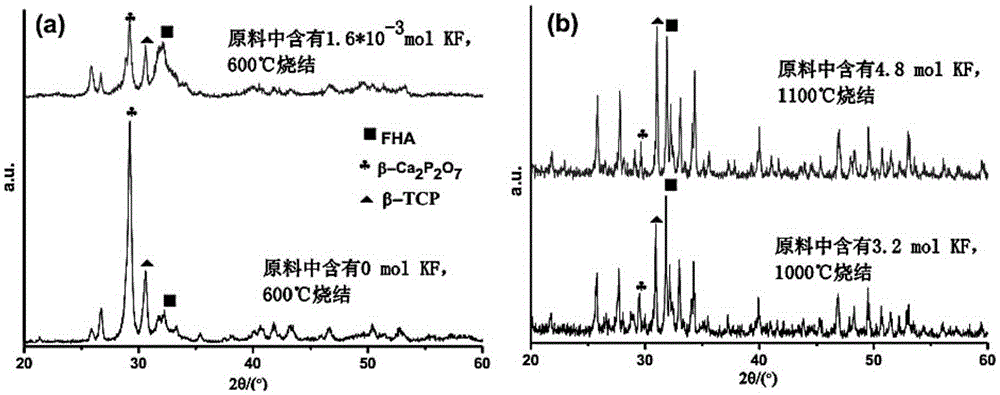
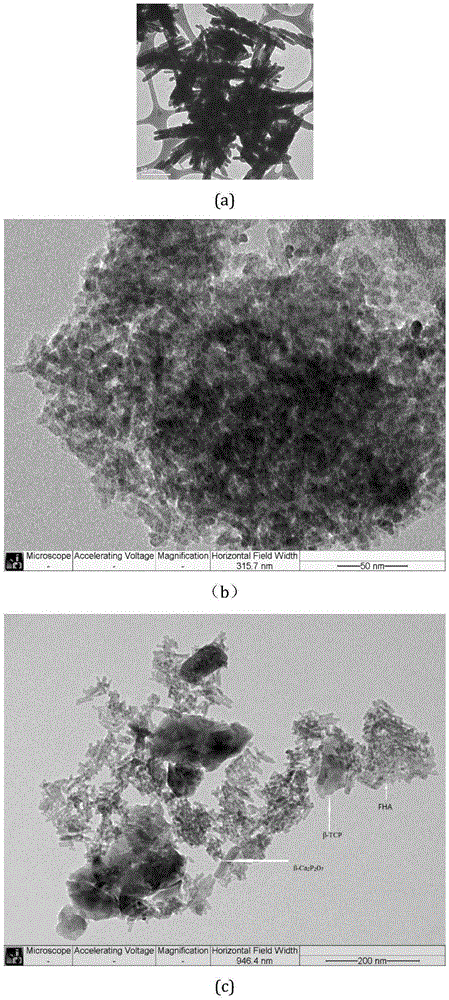
[0024] This embodiment includes the following operations: 0.04mol calcium acetate monohydrate is dissolved in 200mL water, 0.024mol potassium dihydrogen phosphate and 1.6×10 -3 mol potassium fluoride dihydrate was dissolved in 120mL water, the potassium hydroxide solution was used to adjust the pH of the two solutions to 7, and then the KH 2 PO 4 The mixed solution with KF was added dropwise to Ca(CH 3 COO) 2 solution, while closing the reaction vessel to avoid CO 2 Reacted. The pH of the solution was adjusted to be 7 every 1h, electromagnetically stirred for 4h, aged for 24h, and dried at 80°C to obtain the following: image 3 The precursor powder shown in a.
[0025] The precursor powder was sintered at 600°C, the holding time was 2h, and the heating rate from room temperature to 600°C was 10°C / min.
[0026] This embodiment obtains FHA / β-TCP / β-Ca 2 P 2 o 7 Three-phase ceramics, the structure is dispersed and the composition is uniform, such as image 3 As shown in ...
Embodiment 3
[0028] This embodiment includes the following operations: 0.04mol calcium acetate monohydrate is dissolved in 200mL water, 0.024mol potassium dihydrogen phosphate and 3.2×10 -3 mol potassium fluoride dihydrate was dissolved in 120mL water, the potassium hydroxide solution was used to adjust the pH of the two solutions to 7, and then the KH 2 PO 4 The mixed solution with KF was added dropwise to Ca(CH 3 COO) 2 solution, while sealing the reaction vessel to avoid CO 2 Reacted. The pH of the solution was adjusted to 7 every 1 hour, electromagnetically stirred for 4 hours, aged for 24 hours, and then dried at 80° C. to obtain a precursor powder.
[0029] The precursor powder is sintered at 1000°C, the holding time is 2h, and the heating rate from room temperature to 1000°C is 10°C / min.
[0030] This embodiment obtains FHA / β-TCP / β-Ca 2 P 2 o 7 The three-phase ceramic has better biodegradability and higher thermal stability than that of Example 2.
Embodiment 4
[0032] This embodiment includes the following operations: 0.04mol calcium acetate monohydrate is dissolved in 200mL water, 0.024mol potassium dihydrogen phosphate and 4.8×10 -3 mol potassium fluoride dihydrate was dissolved in 120mL water, the potassium hydroxide solution was used to adjust the pH of the two solutions to 7, and then the KH 2 PO 4 The mixed solution with KF was added dropwise to Ca(CH 3 COO)2 solution, while sealing the reaction vessel to avoid CO 2 Reacted. The pH of the solution was adjusted to 7 every 1 hour, electromagnetically stirred for 4 hours, aged for 24 hours, and then dried at 80° C. to obtain a precursor powder.
[0033] The precursor powder is sintered at 1100°C, the holding time is 2h, and the heating rate from room temperature to 1100°C is 10°C / min.
[0034] This embodiment obtains FHA / β-TCP / β-Ca 2 P 2 o 7 Three-phase ceramics, the three-phase structure coexists and is mixed evenly, such as image 3 As shown in c, the sintering stability...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com