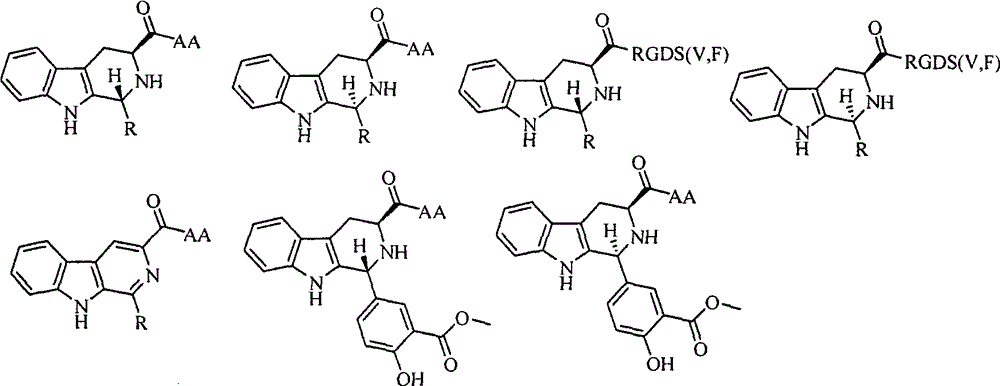
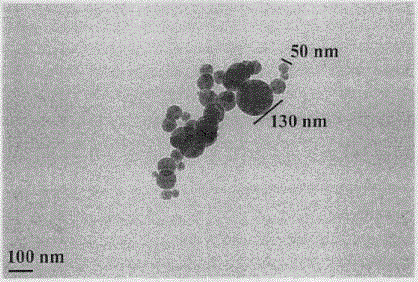
Lys-Glu modified indoloquinolizine, preparation, nano-structure, activity and application thereof
A technology of -lys-glu and quinolizine, which is applied in the field of its anti-tumor cell adhesion, invasion and migration, and the preparation of anti-tumor drugs, can solve the problems of not obtaining anti-tumor and anti-tumor metastatic compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
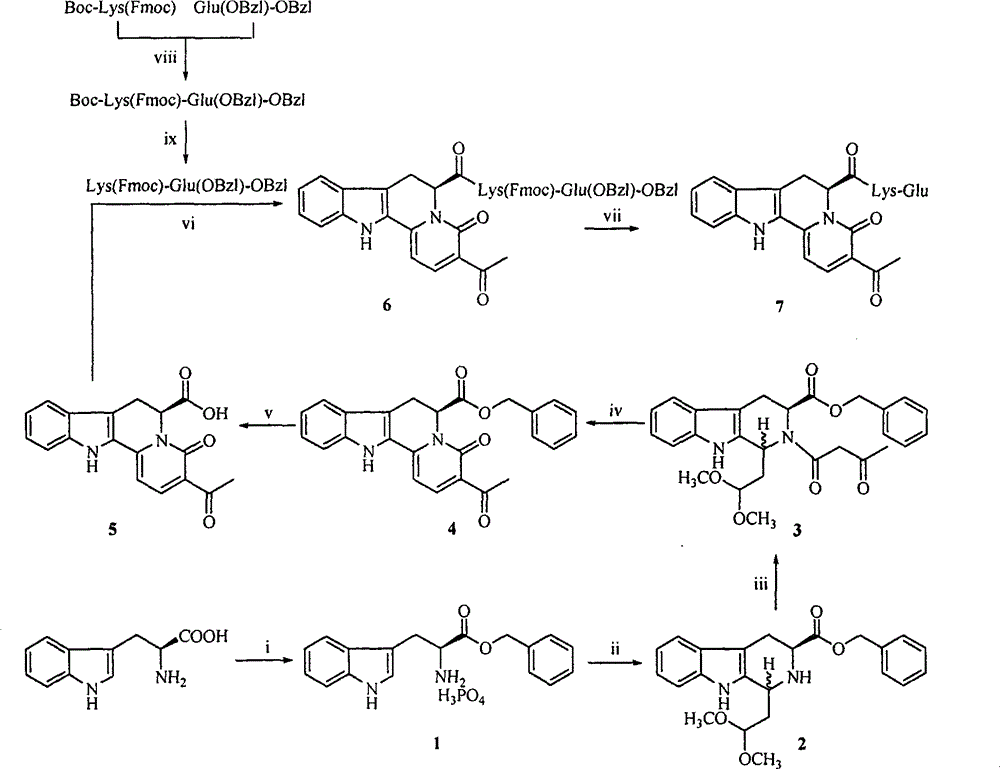
[0026] Embodiment 1 prepares L-tryptophan benzyl phosphate (1)
[0027] Add 20.4g (100mmol) of L-tryptophan, 120mmol of benzyl alcohol and 120mmol of polyphosphoric acid into a 500ml eggplant bottle in turn, install a condenser at the mouth of the bottle, place it in an oil bath and heat to 80°C, react for 72 hours, TL°C shows The starting material disappeared completely, and the reaction was terminated. The reaction solution was cooled to room temperature, and 30 ml of anhydrous ether was added to precipitate a white solid, which was stirred for 2 hours. The reaction solution was filtered under reduced pressure to obtain a white solid, which was washed three times with anhydrous ether to obtain 35.4 g (90.4%) of the target compound. It is a white solid. ESI-MS(m / e): 393.1[M+H] + .
Embodiment 2
[0028] Example 2 Preparation of (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (2)
[0029] 250ml CH 2 Cl 2 Add it to a 500ml eggplant bottle, add 15ml 1,1,3,3-tetramethoxypropane and 14ml trifluoroacetic acid successively under ice bath, stir for 0.5 hours, add 20.0g (68mmol) L-tryptophan benzyl ester, stir at room temperature After 120 hours, the reaction ended. Slowly add saturated Na to the reaction solution with stirring in an ice bath 2 CO 3 aqueous solution, stirred vigorously until the pH of the aqueous layer was 7, separated the dichloromethane layer, and washed the dichloromethane layer with 5% NaHCO 3 aqueous solution and saturated NaCl aqueous solution were extracted and washed 3 times, and the dichloromethane layer was washed with anhydrous NaCl 2 SO 4 Dry, filter under reduced pressure, and concentrate the filtrate to dryness under reduced pressure. The residue is purified by silica gel column chromatography (petro...
Embodiment 3
[0030] Example 3 Preparation of (3S)-1-(2,2-dimethoxyethyl)-2-acetoacetyl-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (3)
[0031]Dissolve 9.38g (23.8mmol) (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester in 200ml Acetone, 2.89ml of diketene and 1.81ml of triethylamine were added under stirring in an ice bath, and stirred at room temperature for 18 hours. TLC showed that the raw materials completely disappeared, and the reaction was completed. Add 10ml of distilled water under stirring in an ice bath, stir for 1 hour, concentrate under reduced pressure to remove acetone, extract the residue 3 times with dichloromethane, combine the dichloromethane layers, and successively wash with 5% NaHCO 3 aqueous solution and saturated NaCl aqueous solution were extracted and washed 3 times, and the dichloromethane layer was washed with anhydrous NaCl 2 SO 4 After drying and filtering under reduced pressure, the filtrate was con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com