Modified sodium alginate and application thereof
A modification technology of sodium alginate, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, blood diseases, etc., can solve the problems of non-degradability, high swelling rate of hydrogel, poor biocompatibility, etc., and achieve Good biocompatibility and biodegradability, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
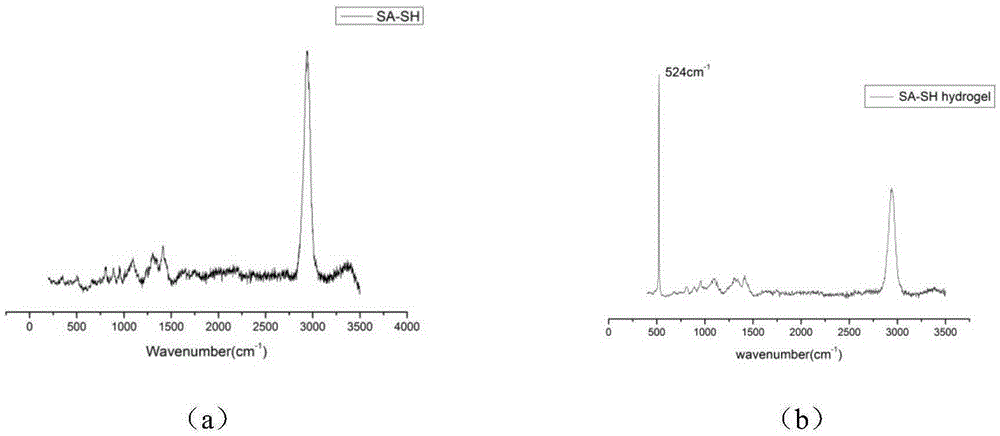
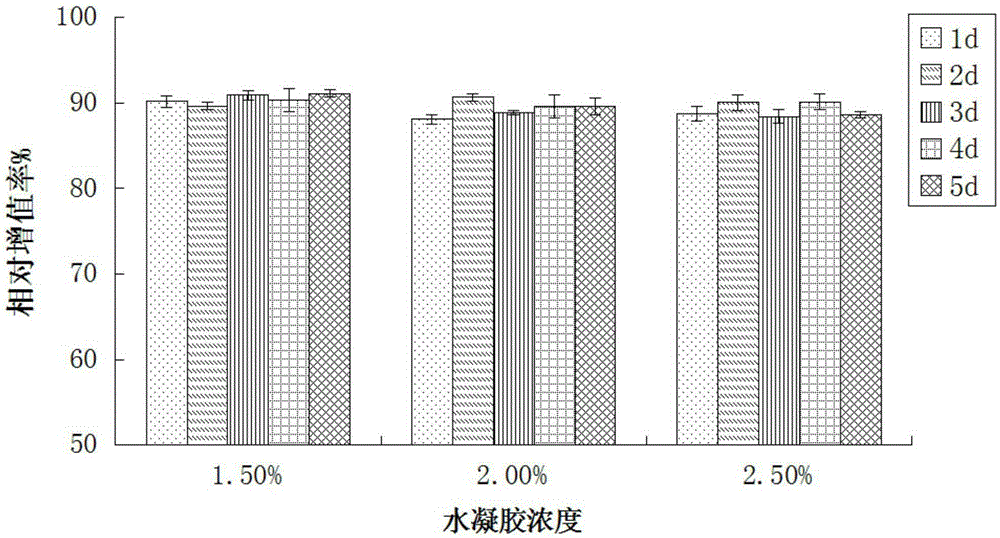
[0036] Weigh 10 g of sodium alginate with a molecular weight of 50 kDa and M / G=1:1, dissolve it in 200 mL of an aqueous solution with pH=3, and prepare a 50 g / L sodium alginate aqueous solution. Dissolve EDC (1g) and NHS (1g) in 5mL of water to prepare a catalyst solution, add the prepared catalyst solution to the above aqueous solution of sodium alginate with a concentration of 50g / L, and stir at room temperature (20°C) for 1 hour, then add L-cysteine methyl ester hydrochloride (2g), heat up to 60°C, continue to stir for 2h, stop stirring, settle with 400mL of ethanol, collect the precipitate by suction filtration, wash the precipitate with 50ml of ethanol respectively Finally, the precipitate was washed three times with 50 mL of ether, and dried in vacuum for 4 hours to obtain thiol-modified sodium alginate (abbreviated as "SA-SH-1"). The mercapto group grafting rate of SA-SH-1 was 10 %.
[0037] Put SA-SH-1 in a ball mill and pulverize it, weigh 0.1g of powdered SA-SH-1 ...
Embodiment 2
[0043] Weigh 10 g of sodium alginate with a molecular weight of 150 kDa and M / G=1:0.5, dissolve it in 200 mL of an aqueous solution with pH=5, and prepare a 15 g / L sodium alginate aqueous solution. Dissolve EDC (2g) and NHS (2g) in 10mL of water to prepare a catalyst solution, add the prepared catalyst solution to the above aqueous sodium alginate solution with a concentration of 15g / L, and stir at room temperature (20°C) for 1 hour, then add L-cysteine methyl ester hydrochloride (4g), heat up to 50°C, continue to stir for 2h, stop stirring, settle with 800mL of ethanol, collect the precipitate by suction filtration, wash the precipitate with 100ml of ethanol respectively Finally, the precipitate was washed five times with 100 mL ether, and dried in vacuum for 12 hours to obtain mercapto-modified sodium alginate (abbreviated as "SA-SH-2"). The mercapto group grafting rate of SA-SH-2 15%.
[0044]Put SA-SH-2 in a ball mill and pulverize it, weigh 0.1g of powdered SA-SH-2 int...
Embodiment 3
[0047] Weigh 10 g of sodium alginate with a molecular weight of 300 kDa and M / G=1:1, dissolve it in 200 mL of an aqueous solution with pH=6, and prepare a 50 g / L sodium alginate aqueous solution. Dissolve EDC (3g) and NHS (3g) in 20mL of water to prepare a catalyst solution, add the prepared catalyst solution to the above aqueous sodium alginate solution with a concentration of 15g / L, and stir at room temperature (20°C) for 3 After 1 hour, add L-cysteine methyl ester hydrochloride (5g), heat up to 40°C, continue to stir for 24h, stop stirring, settle with 1L of ethanol, collect the precipitate by suction filtration, wash the precipitate with 200ml of ethanol respectively Finally, the precipitate was washed three times with 200 mL ether, and dried in vacuum for 16 hours to obtain thiol-modified sodium alginate (abbreviated as "SA-SH-3"). The mercapto group grafting rate of SA-SH-3 was 25 %.
[0048] Put SA-SH-3 in a ball mill and pulverize it, weigh 5g of powdered SA-SH-3 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
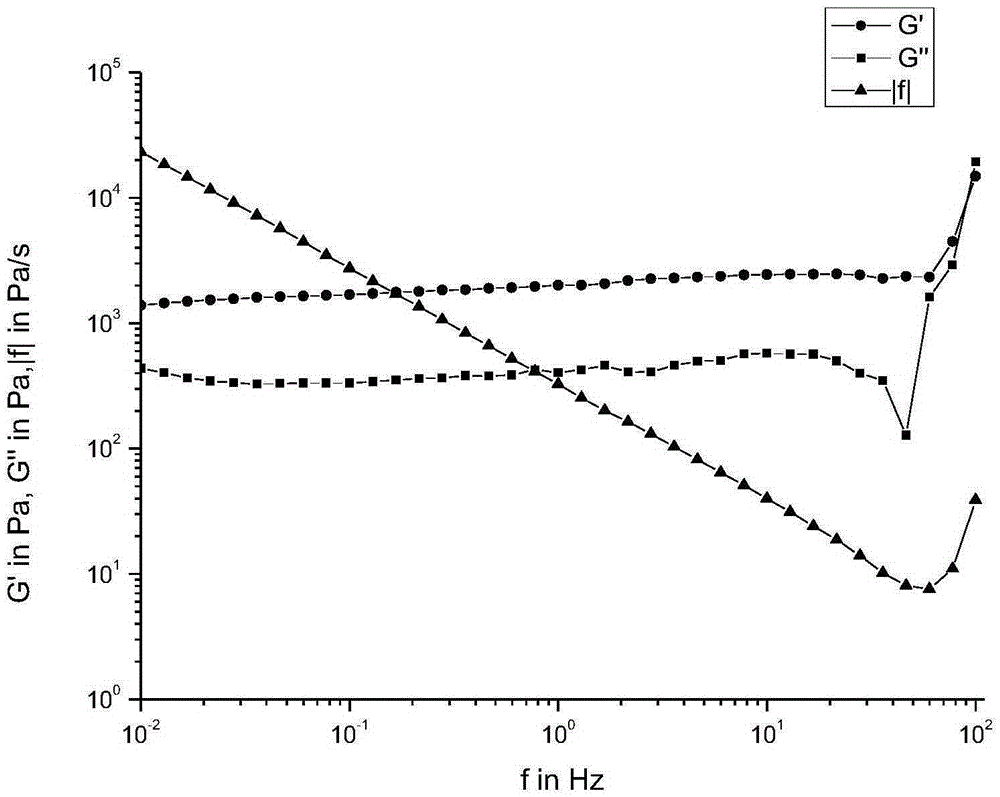
| Storage modulus | aaaaa | aaaaa |
| Loss modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com