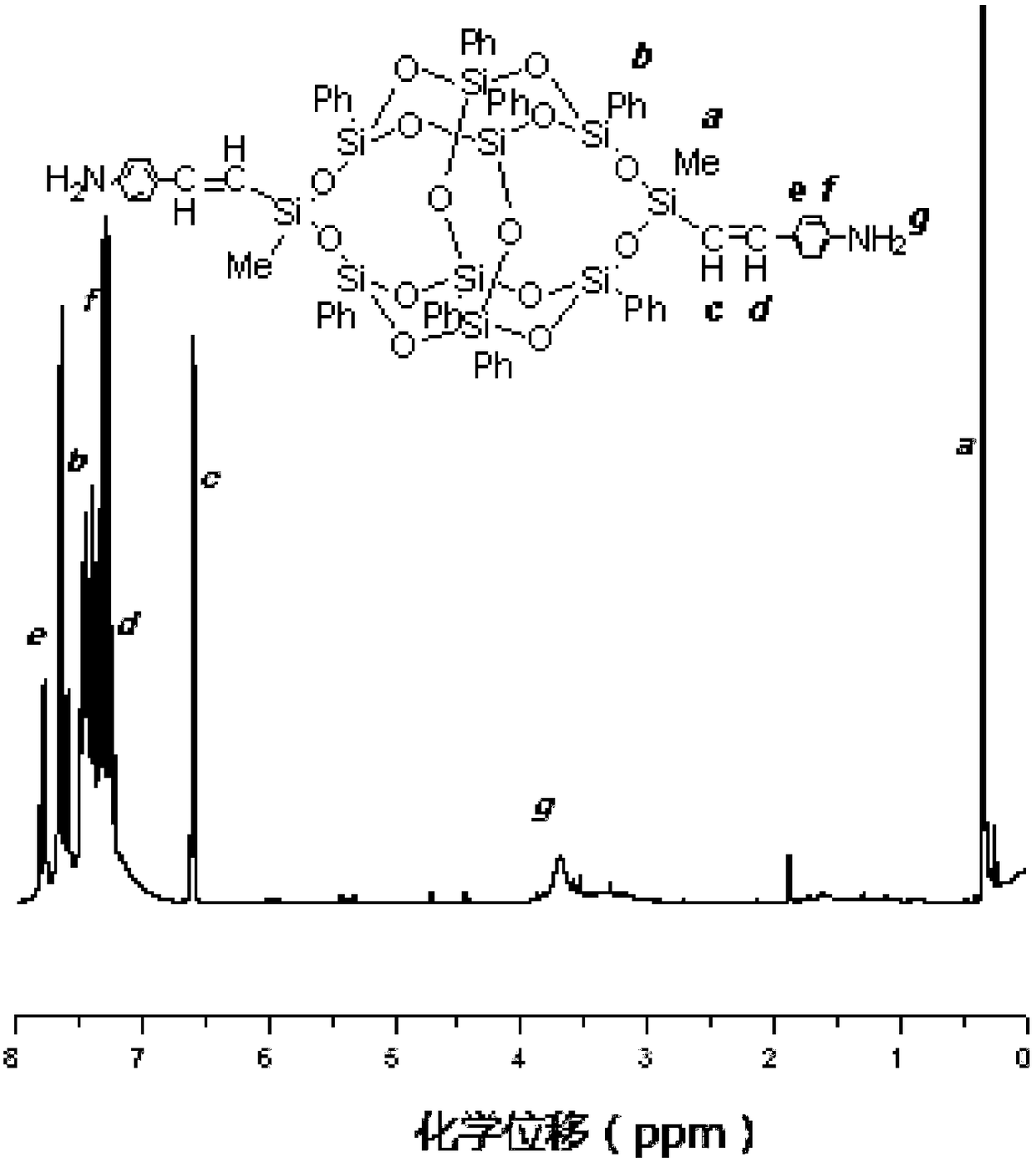
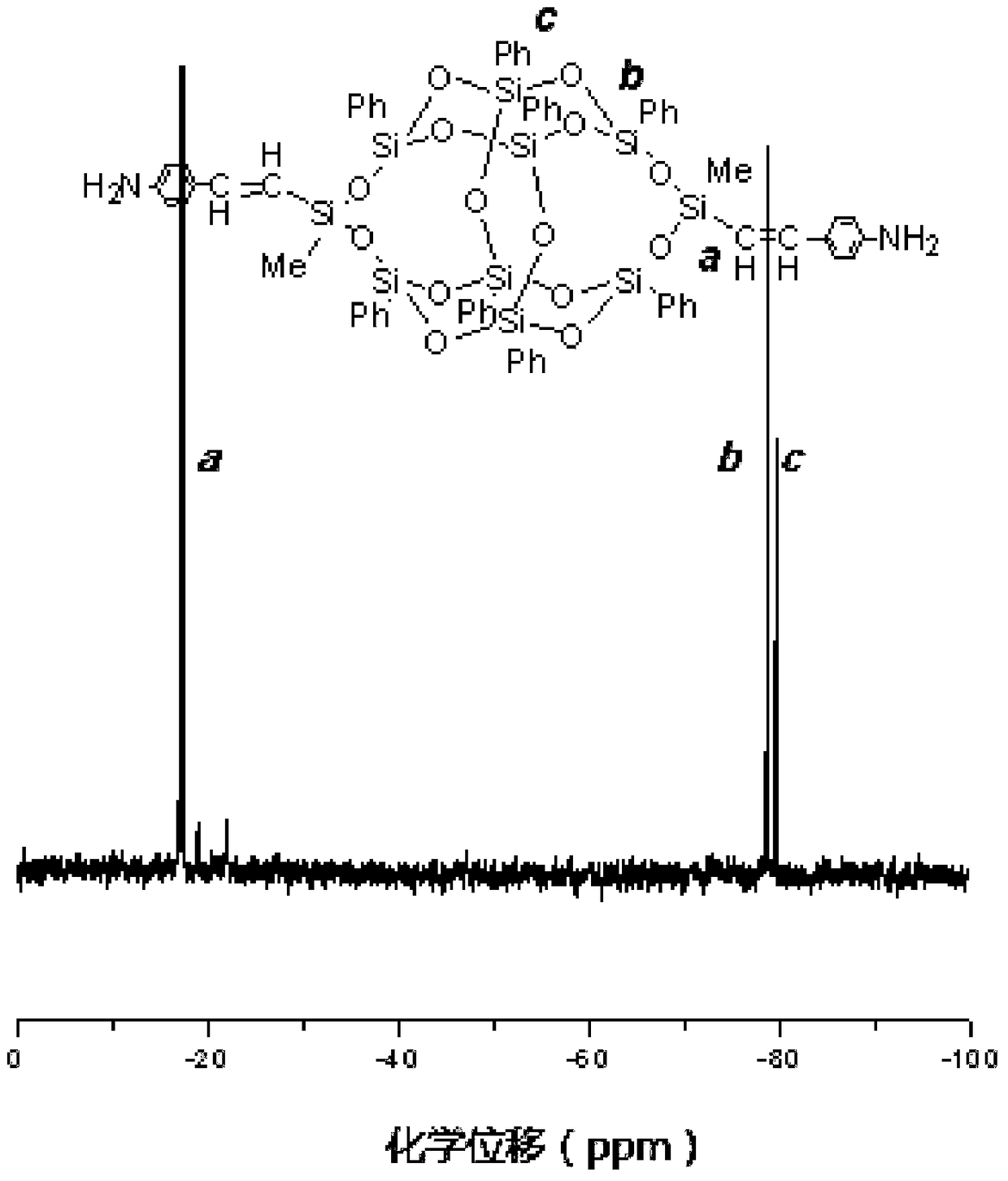
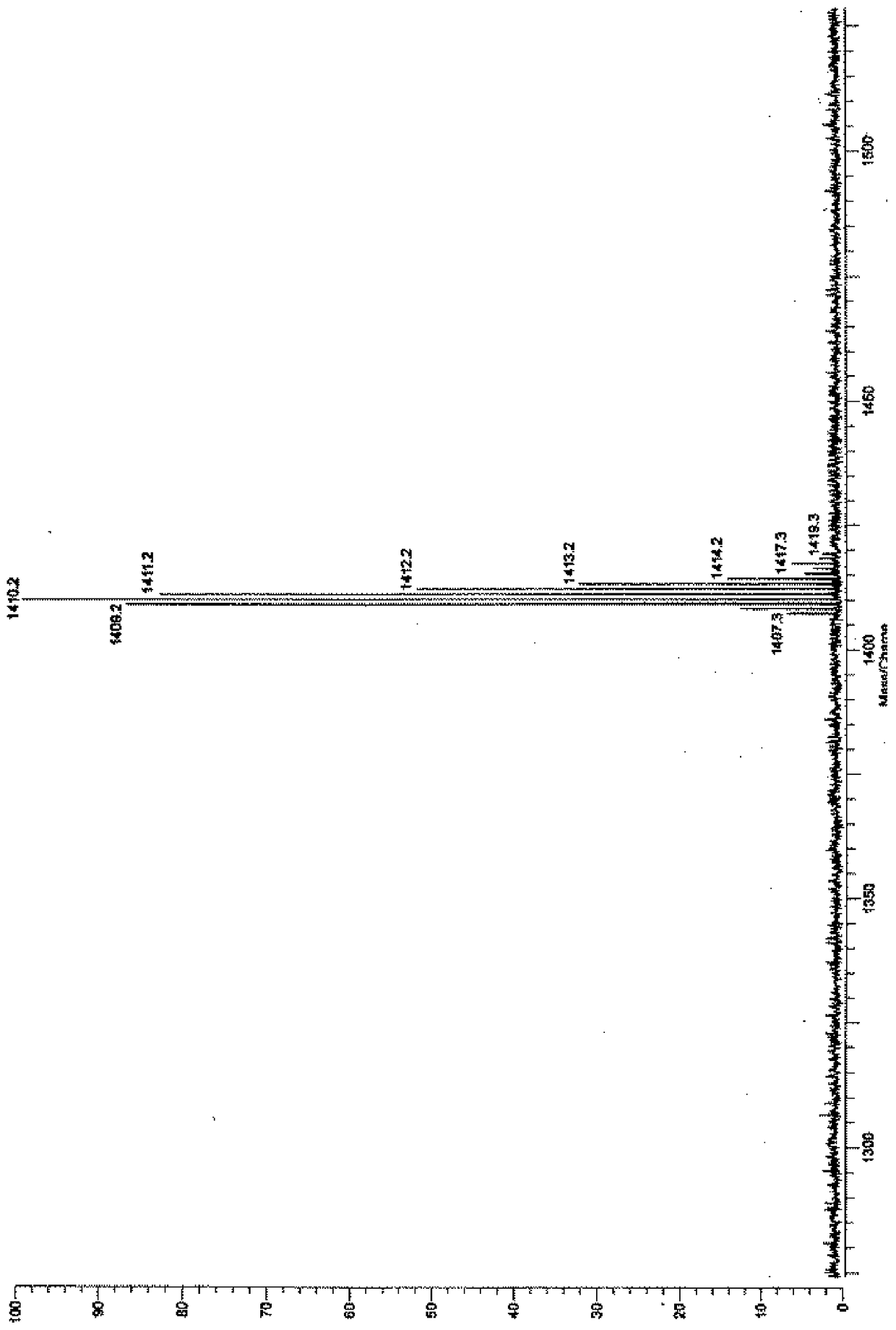
Synthetic method of diaminophenyl double splint cage silsesquioxane
A technology of silsesquioxane and diaminophenyl, which is applied in the field of synthesis of diaminophenyl double splint cage silsesquioxane to achieve the effects of less by-products, simple synthesis steps and low drying degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Preparation of tetrasilanol sodium salt
[0042] In a 500mL single-necked flask equipped with a magnet, add 31.7000g of phenyltrimethoxysilane, 160.0000mL of isopropanol, 3.3300g of deionized water and 4.2800g of sodium hydroxide in sequence, react at 85°C for 4 hours, cool Stirring was continued at room temperature for 15 hours to complete the reaction. All solvents were removed by rotary evaporation, and the product was dried in a vacuum oven at 60° C. for 24 hours to obtain 22.7000 g of a white powdery solid product with a yield of 98.1000%.
[0043] (2) Preparation of terminal alkenyl double splint cage silsesquioxane (3,13-divinyl octaphenyl double splint cage silsesquioxane)
[0044]Add 22.4800g of tetrasilanol sodium salt, 200.0000mL of dry tetrahydrofuran and 5.8300mL of triethylamine into a 500mL one-necked flask, and place it in a low-temperature bath to maintain it at -10°C. 6. 7700g of vinylmethyldichlorosilane was added to the reaction flask at one ti...
Embodiment 2
[0053] (1) Preparation of tetrasilanol sodium salt
[0054] In a 500mL single-necked flask equipped with a magnet, add 31.7000g of phenyltrimethoxysilane, 160.0000mL of isopropanol, 3.3300g of deionized water and 4.2800g of sodium hydroxide in sequence, react at 85°C for 4 hours, cool Stirring was continued at room temperature for 15 hours to complete the reaction. All solvents were removed by rotary evaporation, and the product was dried in a vacuum oven at 60° C. for 24 hours to obtain 22.7000 g of a white powdery solid product with a yield of 98.1000%.
[0055] (2) Preparation of terminal alkenyl double splint cage silsesquioxane (3,13-divinyl octaphenyl double splint cage silsesquioxane)
[0056] Add 22.4800g of tetrasilanol sodium salt, 200.0000mL of dry tetrahydrofuran and 5.8300mL of triethylamine into a 500mL one-necked flask, and place it in a low-temperature bath to maintain it at -10°C. 6. 7700g of vinylmethyldichlorosilane was added to the reaction flask at one t...
Embodiment 3
[0062] (1) Preparation of tetrasilanol sodium salt
[0063] In a 500mL single-necked flask equipped with a magnet, add 31.7000g of phenyltrimethoxysilane, 160.0000mL of isopropanol, 3.3300g of deionized water and 4.2800g of sodium hydroxide in sequence, react at 85°C for 4 hours, cool Stirring was continued at room temperature for 15 hours to complete the reaction. All solvents were removed by rotary evaporation, and the product was dried in a vacuum oven at 60° C. for 24 hours to obtain 22.7000 g of a white powdery solid product with a yield of 98.1000%.
[0064] (2) Preparation of terminal alkenyl double splint cage silsesquioxane (3,13-divinyl octaphenyl double splint cage silsesquioxane)
[0065] Add 22.4800g of tetrasilanol sodium salt, 200.0000mL of dry tetrahydrofuran and 5.8300mL of triethylamine into a 500mL one-necked flask, and place it in a low-temperature bath to maintain it at -10°C. 6. 7700g of vinylmethyldichlorosilane was added to the reaction flask at one t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric loss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com