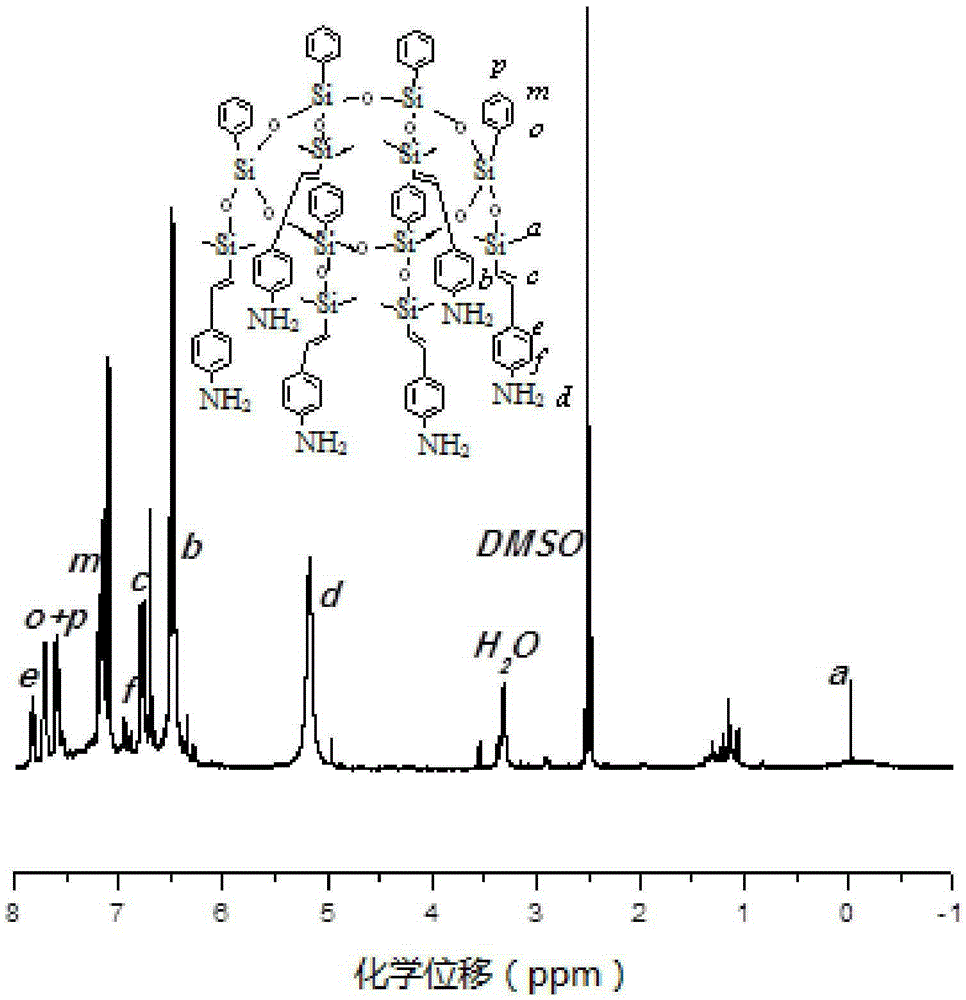
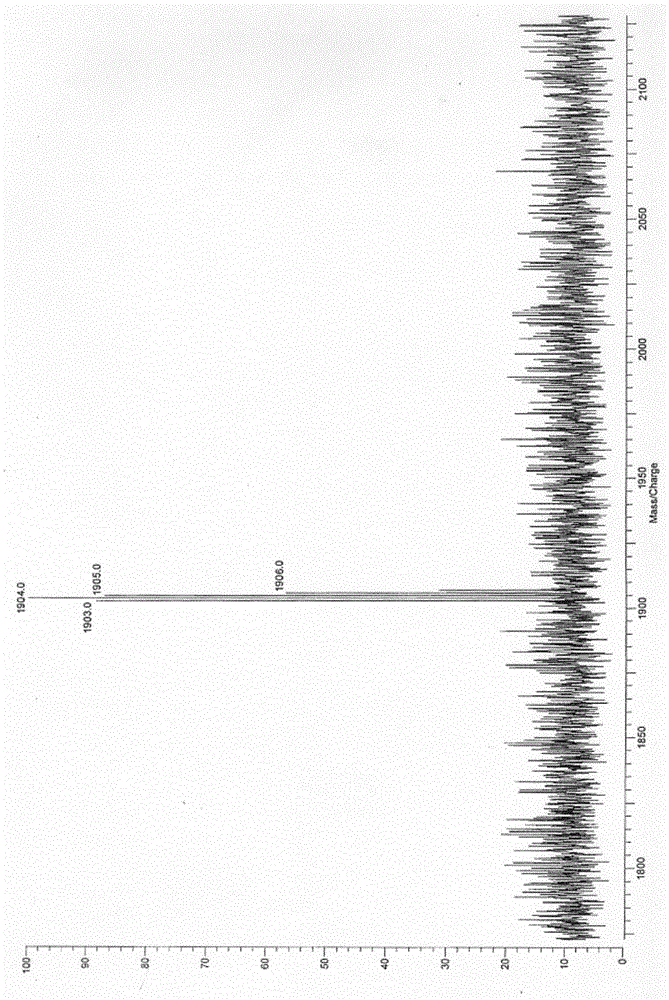
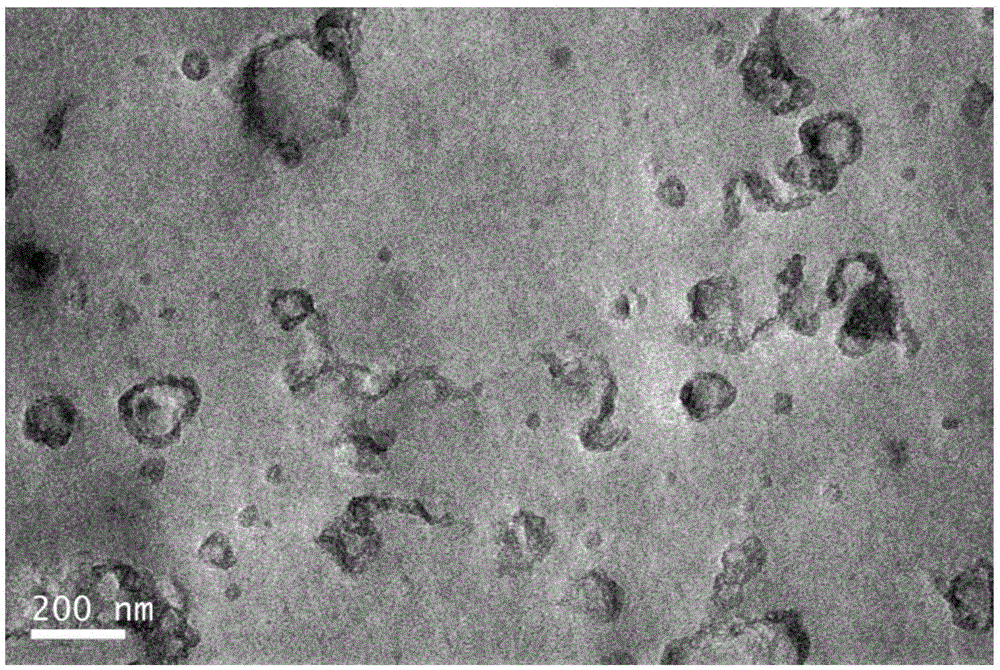
Synthetic method of hexamine phenyl large ring oligomerization silsesquioxane
A technology of polysilsesquioxane and hexaaminephenyl, which is applied in the field of polymer material synthesis to achieve mild reaction conditions, broad application prospects, and extensive functionalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of sodium nickel / salt hexasilicate
[0039] 7.7300g of phenyltrimethoxysilane, 1.8000g of NaOH and 0.7000g of H 2 O was added to 130.0000 mL of n-butanol and refluxed for 1.5 hours. After cooling slightly, add 3.0100g hexaamine nickel dichloride at one time, and then reflux for 2 hours. The NaCl salt was filtered off, part of the n-butanol was removed by rotary evaporation, and the orange crystals were precipitated at 0°C overnight. After being obtained by suction filtration, it was vacuum-dried at 80° C. for 1 hour. Weighed 5.4400g, yield 71.8000%.
[0040] (2) Preparation of alkenyl-terminated macrocyclic oligomerized silsesquioxane (hexavinylhexaphenylcyclohexasiloxane)
[0041] 35.0000 mL of dry toluene, 5.5400 mL of dry pyridine and 10.3200 g of vinyl dimethyl chlorosilane were successively added into a 100 mL flask. After 5 minutes, 1.3000 g of sodium / nickel hexaphenylcyclohexasilate was added. After reacting at room temperature for 24 hours,...
Embodiment 2
[0048] (1) Preparation of sodium nickel / salt hexasilicate
[0049] 7.7300g of phenyltrimethoxysilane, 1.8000g of NaOH and 0.7000g of H 2 O was added to 130.0000 mL of n-butanol and refluxed for 1.5 hours. After cooling slightly, add 3.0100g hexaamine nickel dichloride at one time, and then reflux for 2 hours. The NaCl salt was filtered off, part of the n-butanol was removed by rotary evaporation, and the orange crystals were precipitated at 0°C overnight. After being obtained by suction filtration, it was vacuum-dried at 80° C. for 1 hour. Weighed 5.4400g, yield 71.8000%.
[0050] (2) Preparation of alkenyl-terminated macrocyclic oligomerized silsesquioxane (hexavinylhexaphenylcyclohexasiloxane)
[0051] 35.0000 mL of dry toluene, 5.5400 mL of dry pyridine and 10.3200 g of vinyl dimethyl chlorosilane were successively added into a 100 mL flask. After 5 minutes, 1.3000 g of sodium / nickel hexaphenylcyclohexasilate was added. After reacting at room temperature for 24 hours,...
Embodiment 3
[0057] (1) Preparation of sodium nickel / salt hexasilicate
[0058] 7.7300g of phenyltrimethoxysilane, 1.8000g of NaOH and 0.7000g of H 2 O was added to 130.0000 mL of n-butanol and refluxed for 1.5 hours. After cooling slightly, add 3.0100g hexaamine nickel dichloride at one time, and then reflux for 2 hours. The NaCl salt was filtered off, part of the n-butanol was removed by rotary evaporation, and the orange crystals were precipitated at 0°C overnight. After being obtained by suction filtration, it was vacuum-dried at 80° C. for 1 hour. Weighed 5.4400g, yield 71.8000%.
[0059] (2) Preparation of alkenyl-terminated macrocyclic oligomerized silsesquioxane (hexavinylhexaphenylcyclohexasiloxane)
[0060] 35.0000 mL of dry toluene, 5.5400 mL of dry pyridine and 10.3200 g of vinyl dimethyl chlorosilane were successively added into a 100 mL flask. After 5 minutes, 1.3000 g of sodium / nickel hexaphenylcyclohexasilate was added. After reacting at room temperature for 24 hours,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric loss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com