Aporphine type alkaloid and preparation method thereof
A technology of alkaloids and apophyll, applied in the field of organic synthetic chemistry, can solve the problems of dehydrogenation, no anti-cancer activity, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of 8-acetamido-isocredine:
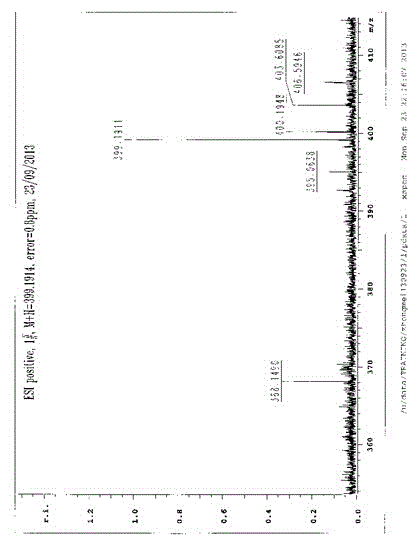
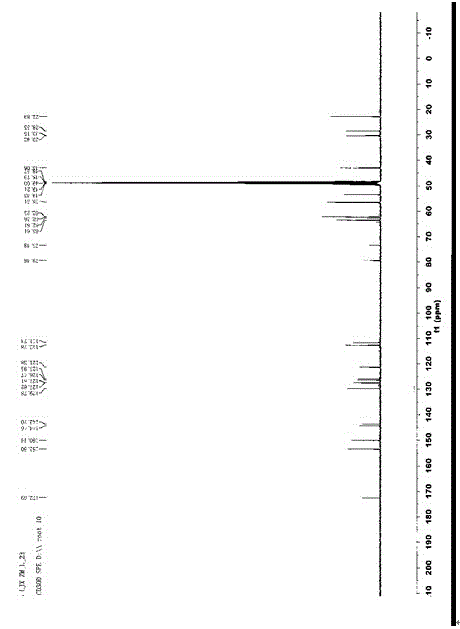
[0025] Dissolve 6g of the reaction raw material 8-amino-isocredine in 20mL of ethyl acetate, stir mechanically to dissolve the reaction raw material completely, add 60mL redistilled acylating reagent acetyl chloride dropwise under mechanical stirring, react for 2h, and identify the reaction by TLC Completely, adjust the pH value of the above reaction product to about 7 with ammonia water, pour the reaction solution into 200mL ice water, extract three times with 300mL chloroform, combine the organic phases, recover the organic solvent, and separate the extract by silica gel column chromatography to obtain 8-acetylamino- Isocridine 5.3g;
[0026]
Embodiment 2
[0027] Embodiment 2: the preparation of 8-acetamido-isocredine:
[0028]The reaction raw material 8-amino-isocredine 6g was dissolved in 20mL chloroform, mechanically stirred, and added dropwise to 60mL redistilled acylating reagent acetyl chloride, reacted for 2h, and the reaction was identified by thin-layer chromatography. Adjust the pH value to about 7, pour the reaction solution into 200mL ice water, extract three times with 300mL chloroform, combine the organic phases, recover the solvent to dryness under reduced pressure, dissolve in 10mL methanol, mix the sample on a silica gel column, and perform column chromatography separation to obtain 8- Acetylamino - isocridine hydrochloride 5.1g;
Embodiment 3
[0029] Embodiment 3: the synthesis of 8-acetamido-isocredine:
[0030] Dissolve 6 g of the reaction material 8-amino-isocreidine in 30 mL of ethyl acetate, mechanically stir to dissolve all the reactants, add 60 mL of freshly distilled acetic anhydride dropwise, react for 2 hours, and adjust the pH value of the above reaction product to 7 or so, pour the reaction liquid into 200mL of ice water, extract three times with 300mL of dichloromethane, combine the organic phase, recover the organic solvent, and separate the extract by silica gel column chromatography to obtain 4.9g of 8-acetylamino-isoxridine;
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com