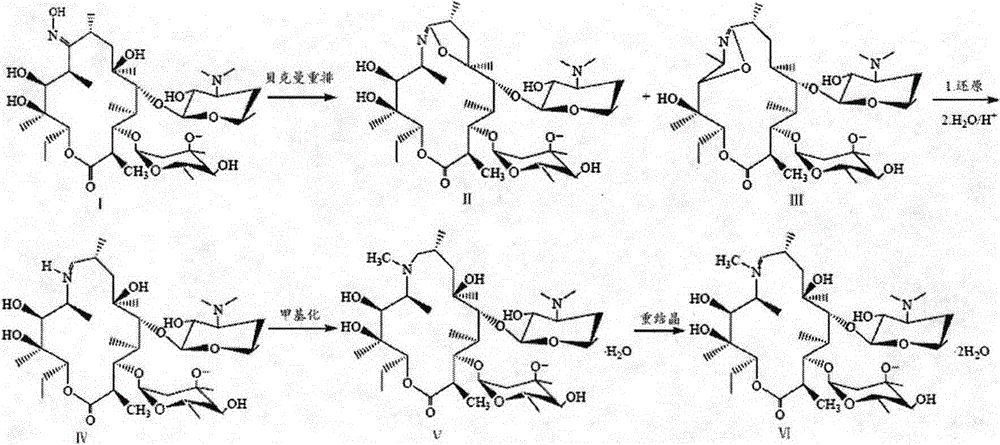
Preparation method for azithromycin intermediate
A technology for azithromycin and intermediates, which is applied in the field of preparation of azithromycin intermediate dihydrohomerythromycin, can solve the problems of low recrystallization yield, incomplete hydrolysis, no industrial application prospect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 200mL of methanol and 18g of erythromycin 6,9-imine ether into a 500mL single-necked bottle, then cool the solution to -10°C, and add 6g of KBH three times in 1 hour 4 , keeping the reaction temperature at -10°C for 6h, the solution was slowly heated to room temperature and concentrated by distillation under reduced pressure. 200 mL of dichloromethane and 200 mL of water were added to the residue, the layers were separated after standing, and the organic layer was concentrated. Add 100mL of acetone and 200mL of water, and add 16g of citric acid monohydrate to it. After the citric acid is dissolved, adjust the pH of the solution to 3 with 6N hydrochloric acid, and stir at room temperature for 1h. Slowly add 20% sodium hydroxide to adjust the pH value to 12, and stir at the same temperature for 1 h. The precipitated crystals were filtered, washed with cold water, and dried overnight at 40° C. to obtain 16.03 g of solid, yield: 89%.
Embodiment 2
[0019] Add 200mL of methanol and 18g of erythromycin 6,9-imine ether into a 500mL single-necked bottle, then cool the solution to 0°C, and add 6g of KBH three times in 1 hour 4 , keeping the reaction temperature at 0°C for 4h, the solution was slowly heated to room temperature and concentrated by distillation under reduced pressure. 200 mL of dichloromethane and 200 mL of water were added to the residue, the layers were separated after standing, and the organic layer was concentrated. Add 100mL of acetone and 200mL of water, and add 16g of citric acid monohydrate to it. After the citric acid is dissolved, adjust the pH of the solution to 2 with 6N hydrochloric acid, and stir at room temperature for 3h. Slowly add 20% sodium hydroxide to adjust the pH value to 11, and stir at the same temperature for 1 h. The precipitated crystals were filtered, washed with cold water, and dried overnight at 40° C. to obtain 13.15 g of solid, yield: 73%.
Embodiment 3
[0021] Add 200mL of methanol and 18g of erythromycin 6,9-imine ether into a 500mL single-necked bottle, then cool the solution to -10°C, and add 3g of KBH three times in 1 hour 4 , keeping the reaction temperature at -10°C for 6h, the solution was slowly heated to room temperature and concentrated by distillation under reduced pressure. 200 mL of dichloromethane and 200 mL of water were added to the residue, the layers were separated after standing, and the organic layer was concentrated. Add 100mL of acetone and 200mL of water, and add 16g of citric acid monohydrate to it. After the citric acid is dissolved, adjust the pH of the solution to 3 with 6N hydrochloric acid, and stir at room temperature for 1h. Slowly add 20% sodium hydroxide to adjust the pH value to 12, and stir at the same temperature for 1 h. The precipitated crystals were filtered, washed with cold water, and dried overnight at 40° C. to obtain 13.73 g of solids, yield: 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com