Carbon-sulfur bond initiator
An initiator and carbon-sulfur bond technology, applied in the field of polymer compounds and polymerization catalysts, to achieve the effects of safe use and storage, low cost, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
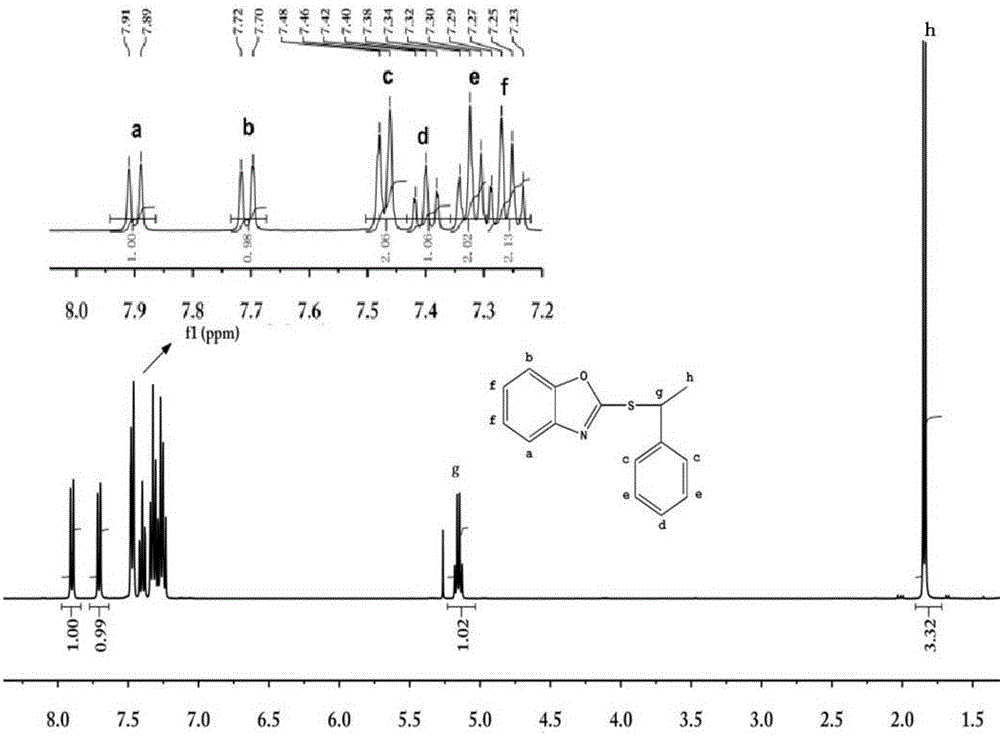
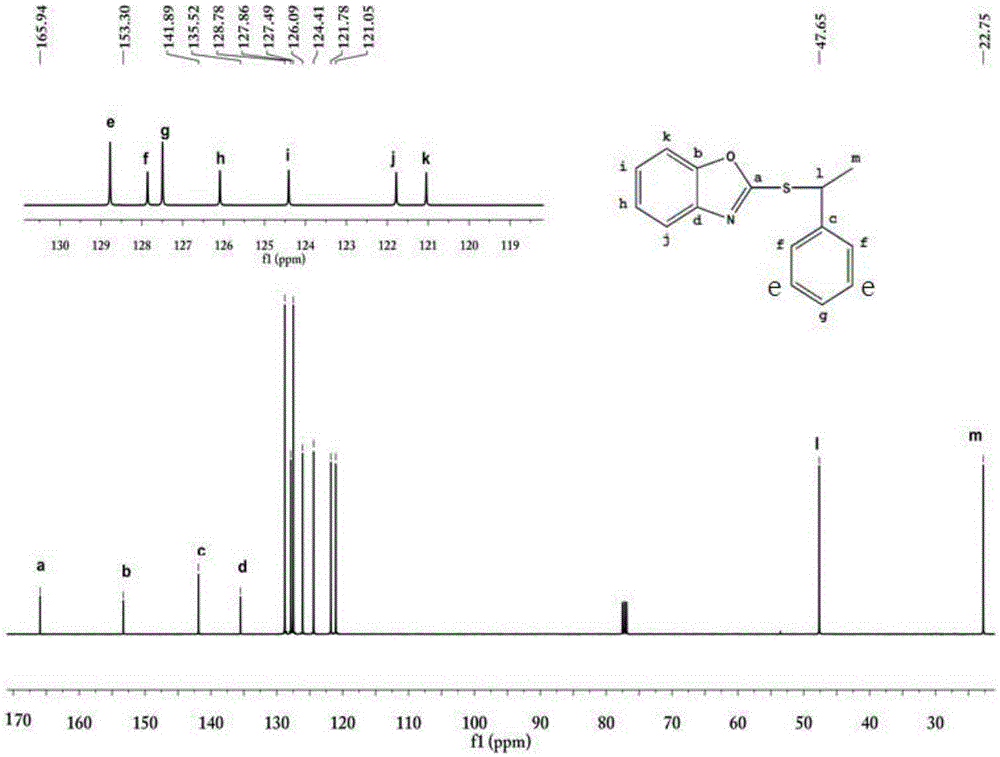
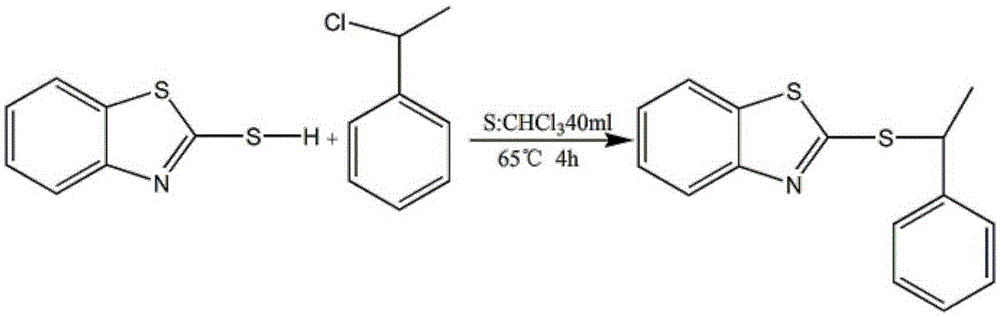
[0021] 3.02g (0.02mol) 2-mercaptobenzoxazole was dissolved in 40ml of dichloromethane solvent, and 2.02g (0.02mol) triethylamine and 1.406g (0.01mol) 1-chlorophenylethane were added under magnetic stirring, Reflux reaction for 4 hours, the solution was cooled to room temperature, and diluted with dichloromethane, the solution was washed with water and saturated brine, the oil phase was dried with anhydrous magnesium sulfate, and the solvent was removed by a rotary evaporator to obtain a crude product. Column separation gave 1.40 g of pure 2-[(1-phenylethyl)-sulfanyl]-benzoxazole.
Embodiment 2
[0023] Dissolve 3.34g (0.02mol) of 2-mercaptobenzothiazole in 40ml of dichloromethane solvent, add 2.02g (0.02mol) of triethylamine and 1.406g (0.01mol) of 1-chlorophenylethane under magnetic stirring, and reflux After reacting for 6 hours, the solution was cooled to room temperature and diluted with dichloromethane, the solution was washed with water and saturated brine, the oil phase was dried with anhydrous magnesium sulfate, and the solvent was removed by a rotary evaporator to obtain a crude product, which was passed through a column Isolation gave 1.78 g of pure 2-[(1-phenethyl)-thio]-benzothiazole.
Embodiment 3
[0027] Take 0.089 g of 2-[(1-phenylethyl)-sulfanyl]-benzoxazole and add it into a polymerization tube containing 3.624 g of styrene monomer and 2 ml of tetrachloroethane, and process it through a vacuum-filling cycle for 3 After that, place it in an oil bath at 120°C for 2, 3, 4, 5, and 6 hours to obtain polystyrene products. The resulting product was compared with the blank solution polymerization reaction group to obtain the following data. See Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com