Quality evaluation method of blood-fat-reducing traditional Chinese medicines on the basis of bio-titer
A technology of biological potency and quality evaluation, applied in biological testing, testing pharmaceutical preparations, material testing products, etc., can solve problems such as the inability to evaluate the characteristics and strength of lipid-lowering activity, and the inability to reflect the strength of biological activity, and achieve perfection. Quality management mode, stable and reliable sensitivity, huge economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
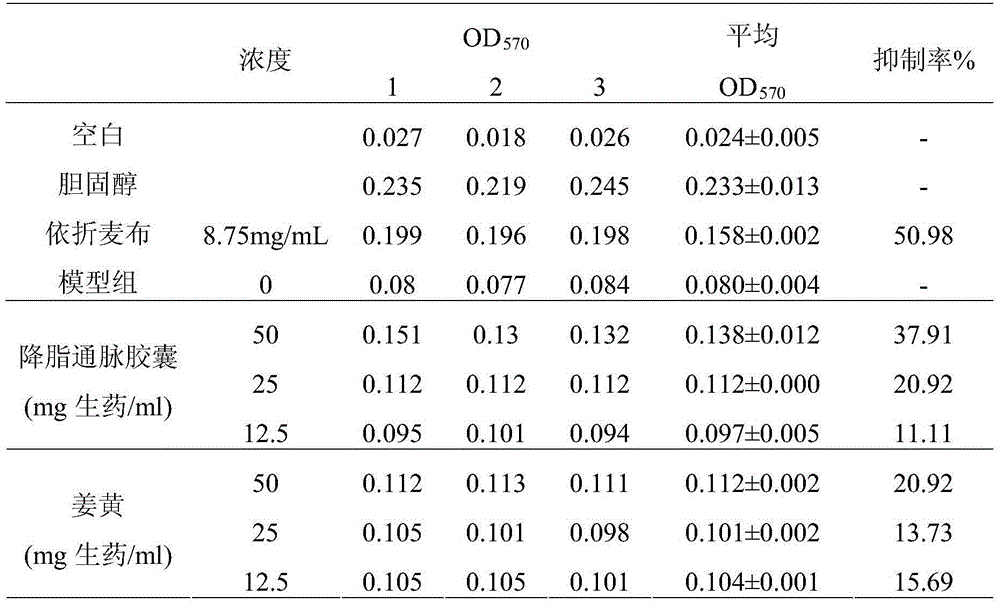
[0083] Firstly, the biological activity of Jiangzhitongmai Capsules and Curcuma longa was determined, and the experimental results are as follows:
[0084] 1. Determination of the biological activity of Jiangzhitongmai capsules and turmeric in inhibiting cholesterol absorption
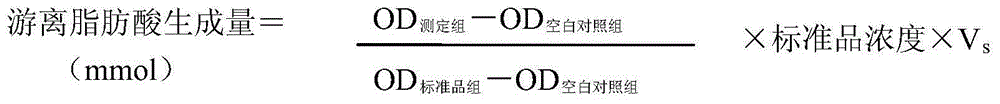
[0085] Add a certain amount of cholesterol and different concentrations of Jiangzhitongmai Capsule test solution, turmeric test solution and ezetimibe solution to differentiated mature Caco-2 cells (differentiated for 17 days) for 24 hours, then absorb the cells The supernatant was used to detect the changes of extracellular free cholesterol to evaluate the effect of Jiangzhitongmai capsules and turmeric on inhibiting cholesterol absorption. Three replicate wells were set up in each group, and the blank control group, model group, cholesterol group and positive control group were set up in parallel, and repeated three times. The cholesterol absorption inhibition rate was calculated according to the fo...
Embodiment 2
[0156] Example 2 Determination of Biological Potency of Jiangzhi Tongmai Capsules in Inhibiting the Intestinal Absorption of Cholesterol
[0157] A. Preparation of reference solution: 3 grains of ezetimibe (10mg), dissolved in 5mL of MEM culture solution, and repeated pipetting and mixing, ultrasonication for 30min, the obtained liquid was filtered through coarse filter paper and 0.22M filter membrane successively, and the final filtrate was collected. That is the ezetimibe solution with a concentration of 6mg / ml, stored at 4°C, and used as a reference solution for later use;
[0158] B. Preparation of test solution: prepare Jiangzhitongmai capsule test solution as previously described;
[0159] C. The test solution and the reference solution are diluted into dose groups of different concentrations with the same dose distance, and the dose distance is 0.5; the solvent used for dilution is MEM medium;
[0160] D. Data processing and potency calculation: respectively measure th...
Embodiment 3
[0164] Example 3 Determination of Biological Potency of Jiangzhitongmai Capsules Inhibiting Triglyceride Intestinal Absorption
[0165] A. Preparation of reference substance solution: use Saini as a reference substance, use deionized water as a solvent, prepare a reference substance solution of corresponding concentration, filter, and set aside;
[0166] B. Preparation of test solution: prepare Jiangzhitongmai capsule test solution as previously described;
[0167] C. The test solution and the reference solution are diluted into dose groups of different concentrations with the same dosage interval, and the dosage interval is 0.5;
[0168] D. Data processing and potency calculation: respectively measure the pancreatic lipase activity inhibition rate obtained by the dosage groups of different concentrations of the test product and the reference product, and compare the test product and the reference product to achieve substantially the same pancreatic lipase activity inhibition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com