Chemical structures of 11 novel phenolic acid compounds with clinical urinary system drug-resistant bacteria resistant activity and application thereof
A technology of chemical structure and drug-resistant bacteria, applied in the fields of organic chemistry, urinary system diseases, organic active ingredients, etc., can solve the problem of unknown active ingredients, etc., and achieve the effect of strong activity and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0045] The preparation of compound described in embodiment case one present invention
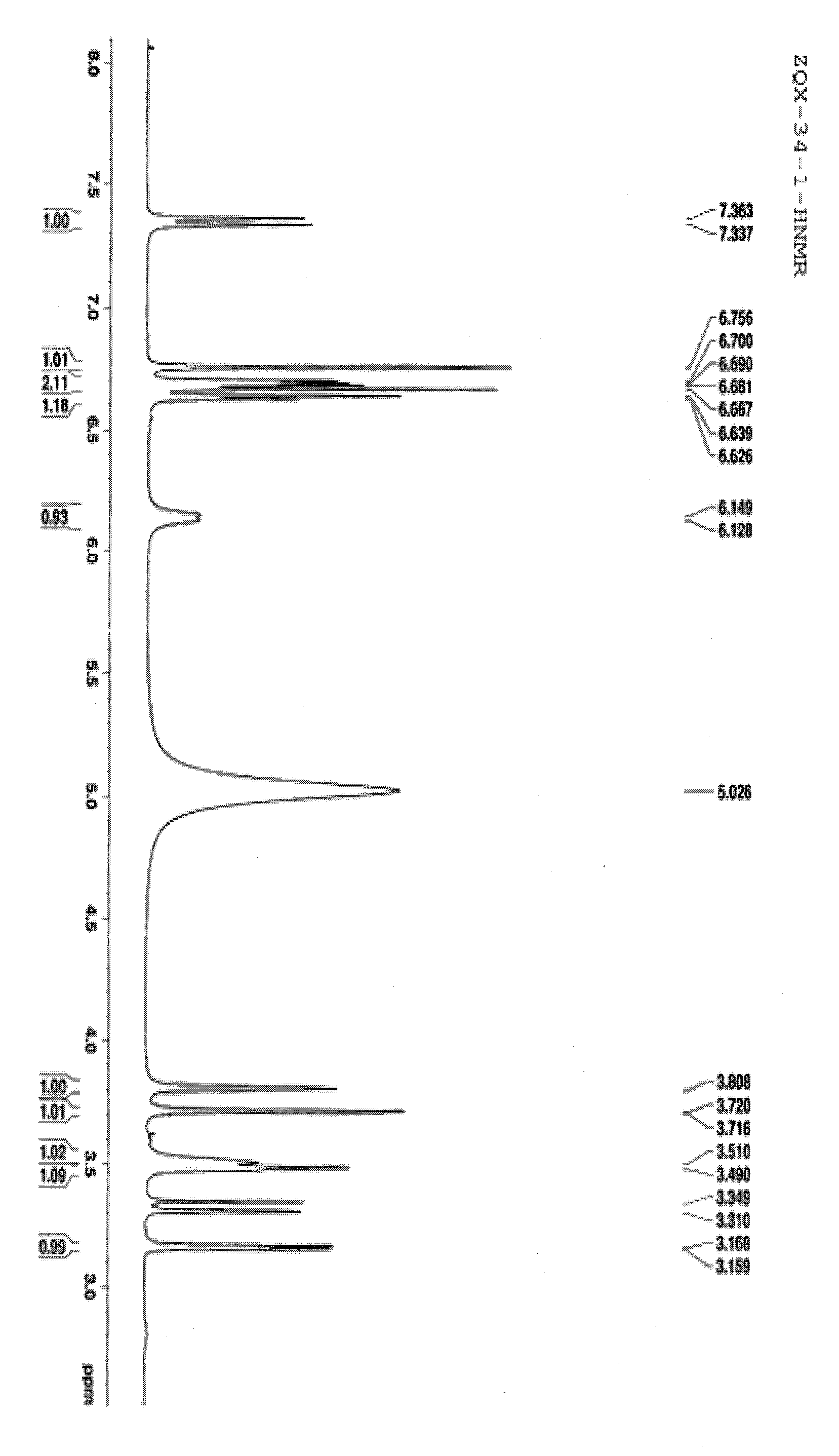
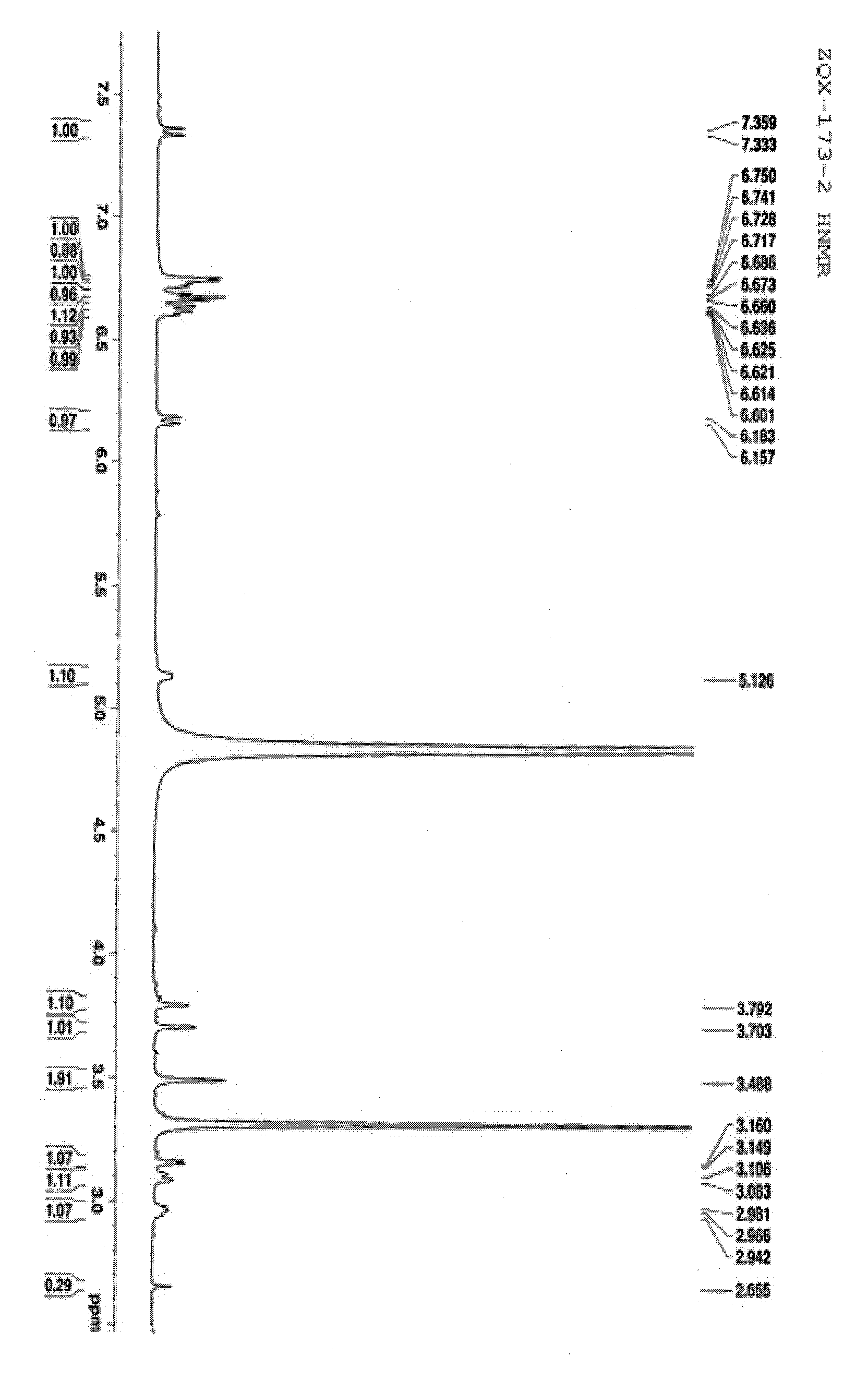
[0046] The effective active part A of cat's whiskers is subjected to macroporous resin chromatography, using ethanol-water (0%, 30%, 60% and 95%) as the eluent to obtain 30% macroporous resin elution part A1, 65% human Porous resin elution part A2 and 95% macroporous resin elution part A3. A1 and A3 were systematically separated. A1 was crudely separated by reverse-phase MCI column chromatography, eluted with methanol-water (0:100-100:0) gradient, and 173 fractions were obtained. Fr.34-42 was separated by gel and ODS column chromatography, eluted with methanol-water, traced by thin layer and combined to obtain 12 fractions, which were separated by reversed-phase HPLC preparative chromatography , to obtain compound 1. Fr.63-83 was separated by gel column, eluted with methanol-water system, and the same components were combined by TLC to obtain 13 fractions. The obtained fractions were sep...
Embodiment example 2
[0047] Embodiment two characterization of the compound of the present invention
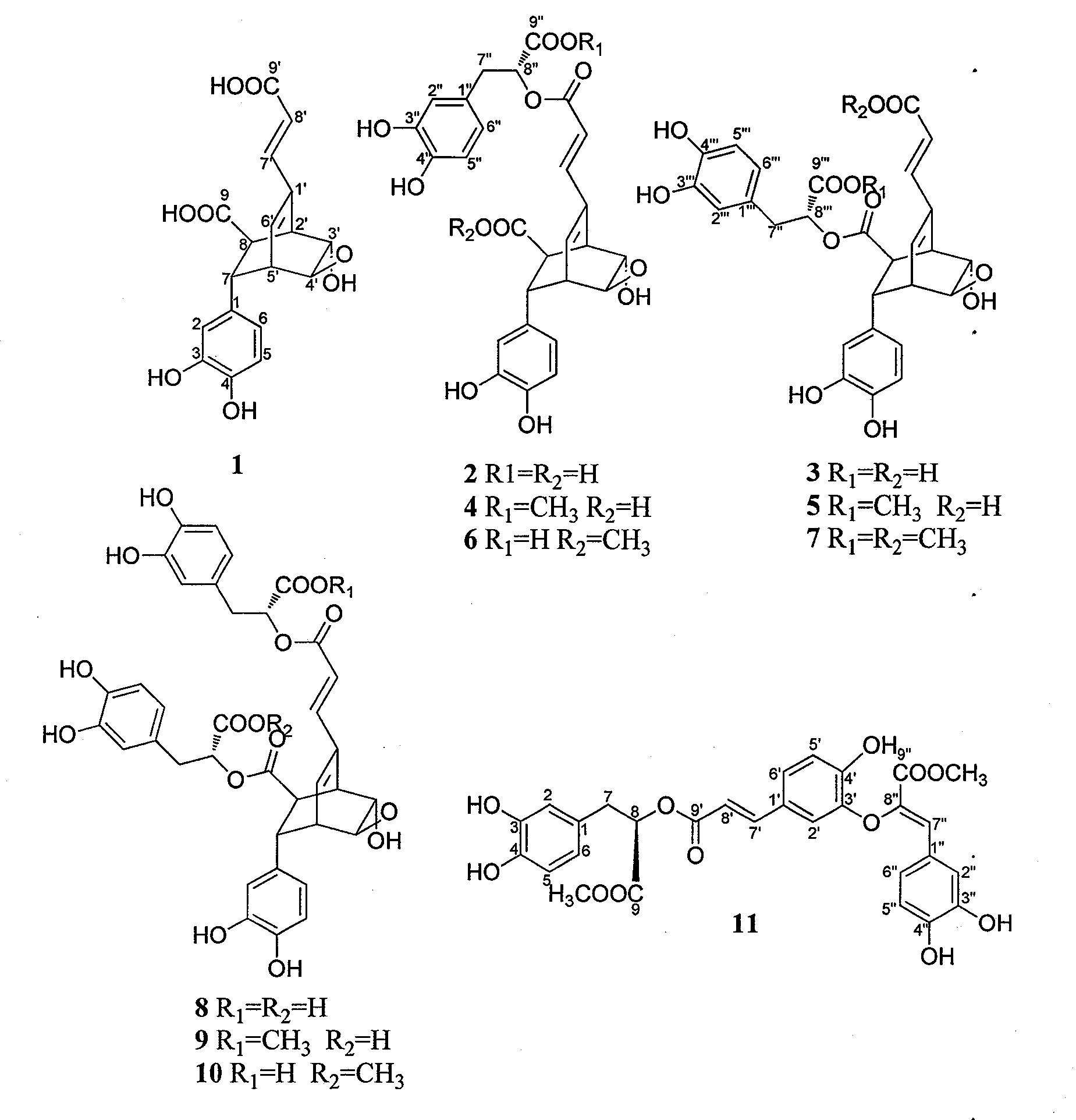
[0048] The structure of compound 1-11 was determined by testing its physical and chemical properties and spectral data.
[0049] The spectral data of compound 1-11 are shown in Table 1-6 and the structure is shown in the following formula.
[0050]
[0051] Table 1 Spectral data of compound 1.2*
[0052]
[0053]
[0054] Spectral data of compound 3-4 in table 2
[0055]
[0056] Table 3 Spectral data of compound 5-6
[0057]
[0058]
[0059] Spectral data of compound 7-8 in table 4
[0060]
[0061] Table 5 Spectral data of compounds 9-10
[0062]
[0063] Table 6 Spectral data of compound 11
[0064]
[0065] *The test solvent is methanol, the hydrogen spectrum data is measured at 600MHz, and the carbon spectrum data is measured at 150MHz.
[0066] The high-resolution mass spectrometry data of compounds 1-11 are shown in Table 7
[0067] Table 7 High resolution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com