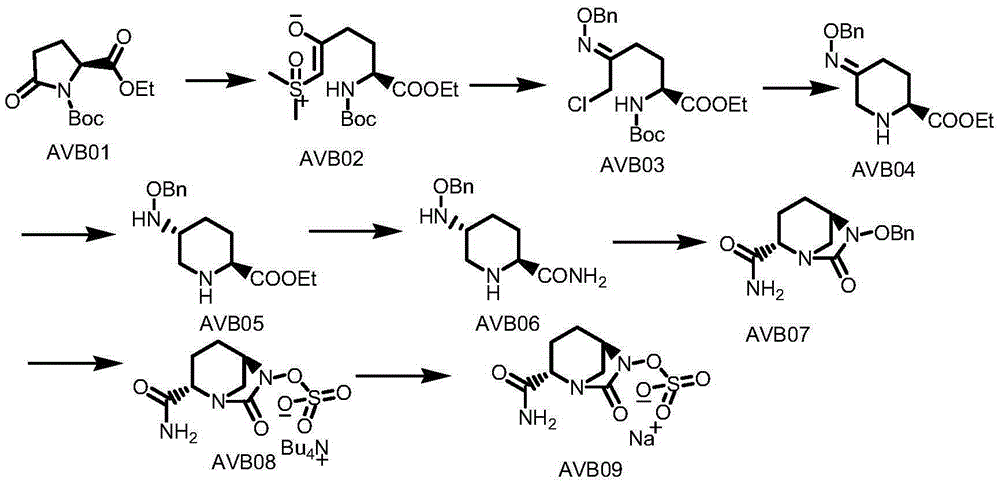
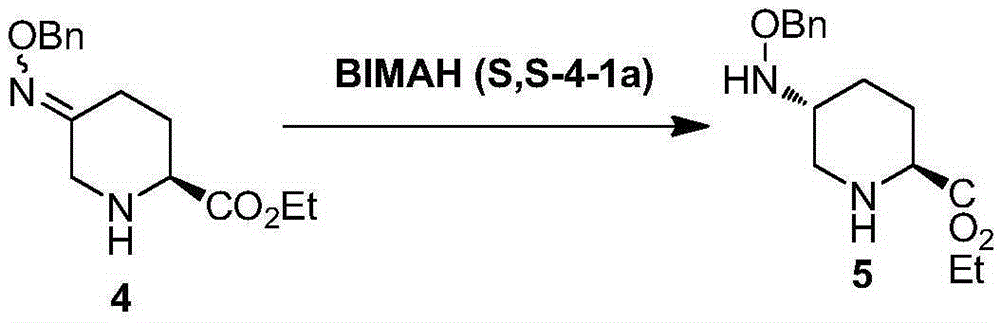
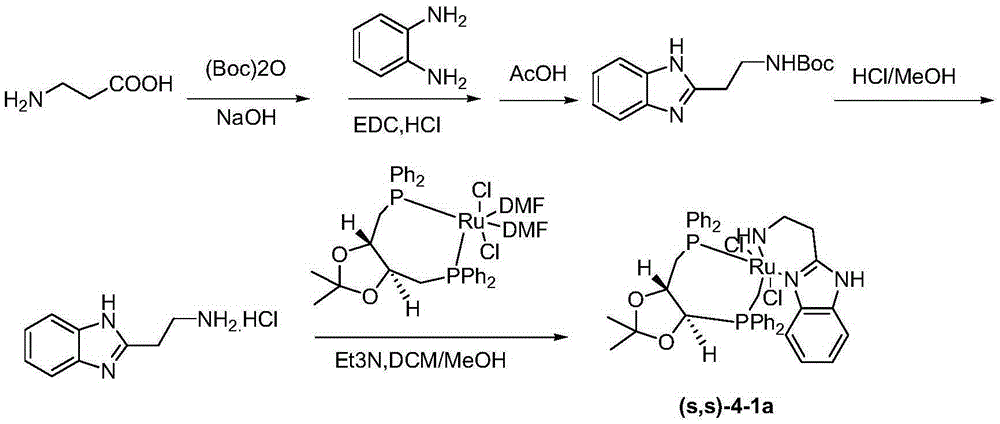
Method for synthesizing avibactam intermediate 5 through asymmetric catalytic hydrogenation method
A technology for catalytic hydrogenation and intermediates, applied in organic chemistry and other directions, can solve the problems of cumbersome post-treatment process and boron pollution, and achieve the effects of easy control of reaction conditions, avoidance of post-treatment, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The solvent B is tetrahydrofuran, the base is potassium tert-butoxide, and the catalyst is β-BIMAH(S,S-4-1a). The molar ratio of the catalyst to the avibactam intermediate 4 was 0.01, and the molar ratio of the base to the catalyst was 100:1.
[0041] Concrete process: get 0.01mol of 5-(benzyloxy) imino) piperidine-2-carboxylate ethyl ester 4 (avibactam intermediate 4) dissolve with tetrahydrofuran 140ml, add the catalyst β-BIMAH of 0.0001mol ( S, S-4-1a), add 0.01mol of potassium tert-butoxide, feed 30bar hydrogen as a reducing agent, and stir at 30°C for 16h. Suction filtration, rotary evaporation to remove the solvent to obtain the off-white solid powder of product 5-(benzyloxy)amino)piperidine-2-carboxylate ethyl ester 5 (avibactam intermediate 5). The product purity is 99.5%, and the yield is 89.9%.
Embodiment 2
[0043] The solvent B is tert-butanol, the base is sodium hydroxide, and the catalyst is β-BIMAH (S, S-4-1a). The molar ratio of the catalyst to the avibactam intermediate 4 was 0.1, and the molar ratio of the base to the catalyst was 1:1.
[0044] Specific process: Take 0.1mol of ethyl 5-(benzyloxy)imino)piperidine-2-carboxylate and dissolve it in 140ml of tetrahydrofuran, add 0.01mol of catalyst β-BIMAH (S, S-4-1a), add 0.01 mol of sodium hydroxide was injected into 25 bar of hydrogen, and stirred at 60°C for 16 hours. Suction filtration, rotary evaporation to remove the solvent to obtain the off-white solid powder of the product ethyl 5-(benzyloxy)amino)piperidine-2-carboxylate. The product purity is 99%, and the yield is 88.9%.
Embodiment 3
[0046] The solvent B is tetrahydrofuran, the base is potassium tert-butoxide, and the catalyst is β-BIMAH(S,S-4-1a). The molar ratio of the catalyst to the avibactam intermediate 4 was 0.05, and the molar ratio of the base to the catalyst was 50:1.
[0047] Specific process: Dissolve 0.01mol of ethyl 5-(benzyloxy)imino)piperidine-2-carboxylate in 140ml of tetrahydrofuran, add 0.025mol of catalyst β-BIMAH(S,S-4-1a), Add 0.01 mol of potassium tert-butoxide, inject 25 bar of hydrogen, and stir at 60° C. for 16 h. Suction filtration, rotary evaporation to remove the solvent to obtain the off-white solid powder of the product ethyl 5-(benzyloxy)amino)piperidine-2-carboxylate. The product purity is 99.1%, and the yield is 88.8%.
[0048] Compared with the prior art, the present invention has the following advantages:
[0049] First, with traditional NaBH 4 Compared with the reduction process, the cumbersome post-treatment is avoided, and the environmental pollution caused by bor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com