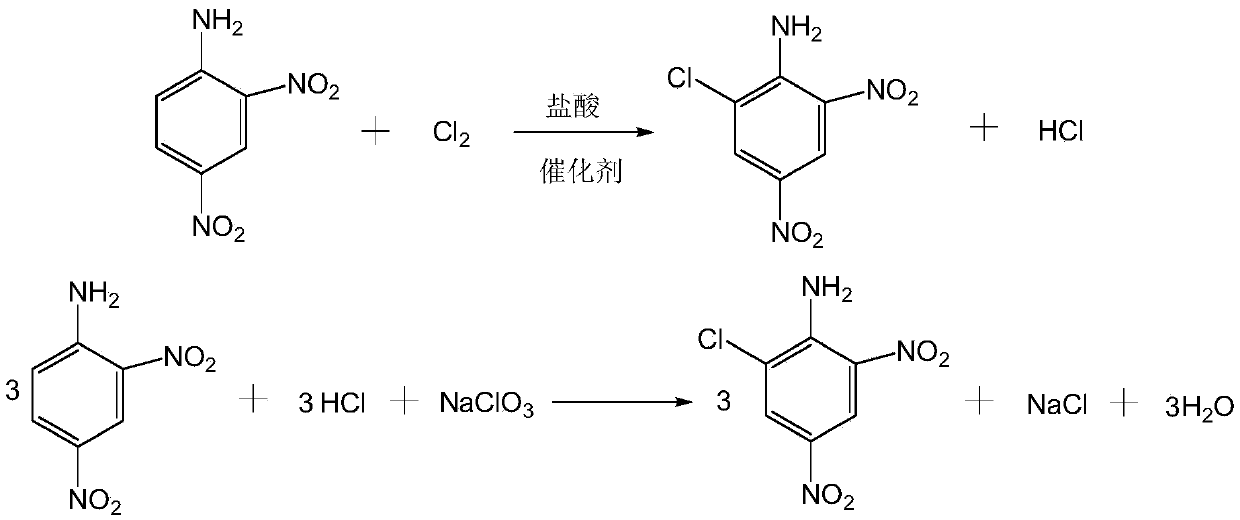
Synthetic technology for 2-chloro-4,6-dinitroaniline
A technology of dinitroaniline and synthesis process, which is applied in the preparation of amino compounds, organic compounds, organic chemistry, etc. It can solve the problems of high acid content in mother liquor wastewater, fewer batches of mother liquor that can be recycled, and increased wastewater discharge. , to achieve significant energy saving and emission reduction, reduce waste water discharge, and use less acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
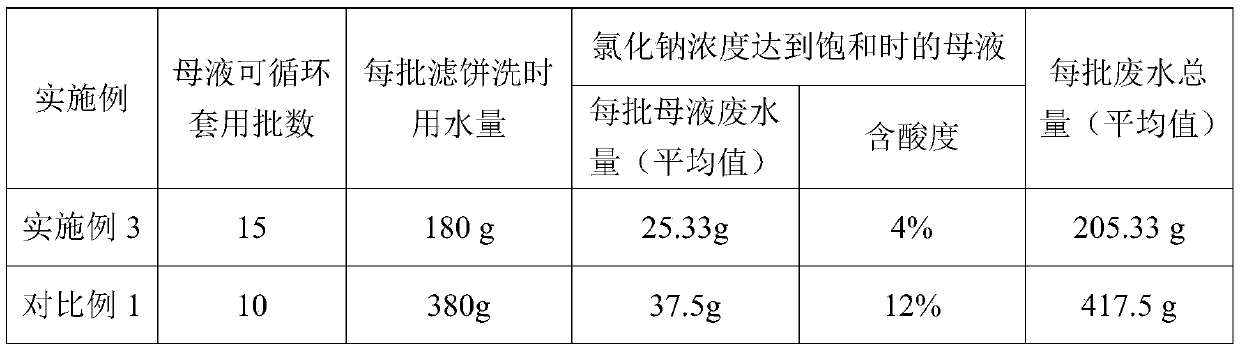
[0028] Add 350 g of 5% hydrochloric acid and 0.4 g of 4-dimethylaminopyridine into a 500 ml three-necked flask with a stirrer and a thermometer, and stir for 15 min; after the stirring time is up, add finely ground 2,4-dinitroaniline 73.2 g, after beating at 25°C for 1 hour, slowly introduce 14.2g of chlorine gas, the time of chlorine gas flow is controlled at 2-3 hours, the temperature of chlorine flow is controlled at 30-35°C, after the chlorine gas is passed, the temperature is raised to 55°C and 27% of Sodium chlorate solution 34.18g, dropwise time is 1h, reaction temperature is controlled at 55~60°C, keep warm for 2h after dropwise addition, then filter, filter cake is washed with 2% liquid caustic to neutrality, obtains 6-chloro- 2,4-dinitroaniline, purity 97.5%, yield 95.6%, mother liquor is collected and applied mechanically to the next batch of reactions, according to the above steps, when the mother liquor is continuously applied mechanically for 15 batches, the conce...
Embodiment 2
[0030] Add 350g of 6% hydrochloric acid and 0.4g of sodium dodecylbenzenesulfonate into a 500ml three-necked flask with a stirrer and a thermometer, and stir for 15 minutes; after the stirring time is up, add finely ground 2,4-dinitro 73.2g of aniline, after beating at 25°C for 1 hour, slowly feed 11.36g of chlorine gas, the time of chlorine gas flow is controlled at 2-3 hours, the temperature of chlorine flow is controlled at 35-40°C, after the completion of chlorine gas flow, the temperature is raised to 50°C, and 27 % sodium chlorate solution 39.96g, the time for dropping is 1h, the reaction temperature is controlled at 50~55°C, after the dropwise addition is completed, it is incubated for 2h, then filtered, and the filter cake is washed to neutrality with 2% liquid alkali to obtain 6- Chloro-2,4-dinitroaniline, the purity is 96.5%, the yield is 95.8%. The mother liquor is collected and applied to the next batch of reactions. According to the above steps, when the mother liq...
Embodiment 3
[0032] Add 350g of 3% hydrochloric acid and 0.4g of dispersant MF into a 500ml three-neck flask equipped with a stirrer and a thermometer, and stir for 15min; after the stirring time is up, add 54.9g of 2,4-dinitroaniline. After beating at 30°C for 1 hour, slowly feed 12.78g of chlorine gas, the time of chlorine gas flow is controlled at 2-3 hours, the temperature of chlorine gas flow is controlled at 30-35°C, after the chlorine gas is passed, the temperature is raised to 55°C and the concentration of mass percentage is 27. % sodium chlorate solution 20.19g, the dropping time is 1h, and the reaction temperature is controlled at 55-60°C. After the dropping is completed, keep warm for 2h, then filter, and wash the filter cake with water until neutral to obtain 6-chloro-2,4 -Dinitroaniline, purity 96.3%, yield 96.0%, mother liquor is collected and applied mechanically to the next batch of reactions, according to the above steps, when mother liquor is continuously applied mechanica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com