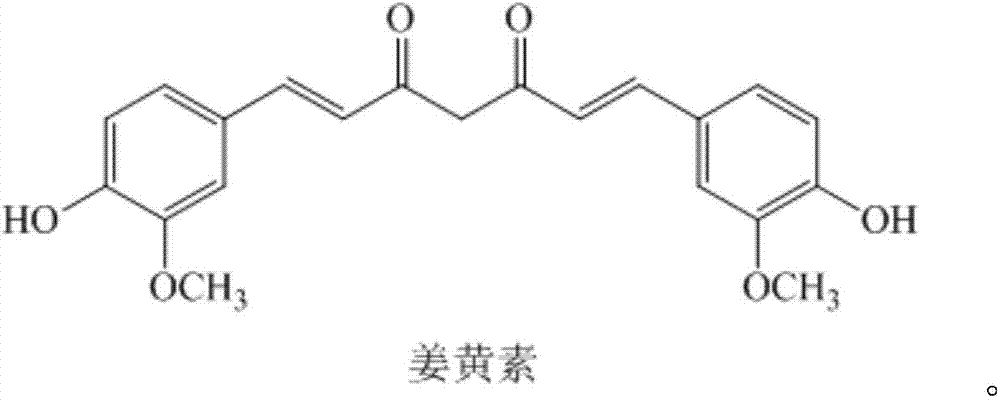
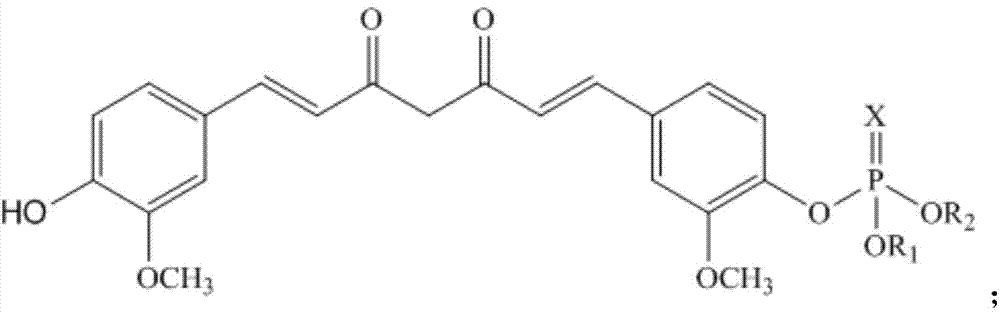
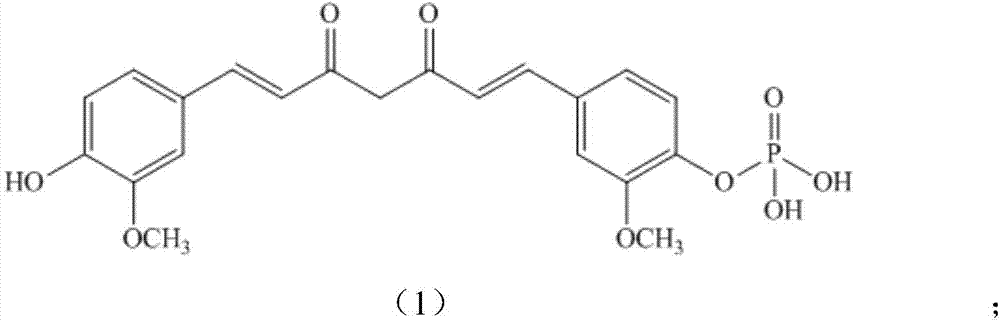
A kind of curcumin phosphate compound, preparation method and application
A technology of curcumin phosphate and compound, applied in the field of curcumin phosphate compound, can solve the problems of fast metabolism, low bioavailability, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083]
[0084] Dissolve 1.84g (0.005mol) of curcumin (0.005mol) and 2.02g (0.02mol) of triethylamine in 30mL of dichloromethane to obtain a solution, lower the solution to 0°C to 5°C, and add dichlorophosphoric acid dropwise while maintaining the temperature Methyl ester 1.45g (0.008mol) in dichloromethane solution 5mL, after the dropwise addition is completed, rise to 20°C and react at this temperature for 24h, stop the reaction to obtain a mixed product, and evaporate the solvent in the mixed product to dryness under reduced pressure to obtain a yellow The oily liquid was subjected to silica gel column chromatography (volume ratio of petroleum ether: acetone = 15:1) to obtain 0.84 g of the product Cur-4 with a yield of 35.1%. Melting point (m.p): 81-83°C. 1 H-NMR (CDCl 3,400MHz)δ:3.890(s,6H),3.903(s,3H),3.916(s,3H),5.816(s,1H),6.464-6.527(m,2H),6.907(d,1H),7.036 -7.113 (m, 4H), 7.287 (d, 1H), 7.546-7.608 (m, 2H); LC-MS m / z: 477 [M+1].
[0085] Dissolve 0.48 g (1 mmol)...
Embodiment 2
[0087] Dissolve 0.45g (1mmol) of Cur-1 prepared in Example 1 in 20mL of ethanol and cool to 0°C to 5°C, keep the temperature and add 0.08g (2mmol) of sodium hydroxide solution 2mL dropwise, and continue the reaction for 30min , the reaction was stopped, and a large amount of yellow solid was precipitated; the solid was filtered and washed with ethanol at 0°C to 5°C to obtain a precipitate, and then the precipitate was vacuum-dried to obtain the product Cur-30.41g, with a yield of 83.3%. m.p.: 160-161°C.
Embodiment 3
[0089] Dissolve 0.48 g (1 mmol) of Cur-4 prepared in Example 1 in 10 mL of acetone, add dropwise 1.00 mL of a solution of 0.23 g (1.50 mmol) of sodium iodide in acetone at 0° C. to 5° C. Stirring at 20°C for 48 hours, a solid precipitate was obtained after the reaction was complete, which was filtered and washed with acetone to obtain 0.37 g of the product Cur-2, with a yield of 75.1%. m.p.: 143-145°C. 1 H-NMR (400Hz, D 2 O)δ: 3.819(s, 3H), 3.882(s, 3H), 3.912(s, 3H), 5.831(s, 1H), 6.406-6.553(m, 2H), 6.825-7.108(m, 5H), 7.269 (d, 1H), 7.525-7.623 (m, 2H); LC-MS m / z: 451 [M-Na].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com