New anti-embolism medicine using small peptide arginine-glycine-aspartic acid (RGD) as leading compound
A compound and leading technology, applied in the field of antithrombotic linear small peptide molecules, to achieve the effect of small molecular weight, strong platelet aggregation activity, and no immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
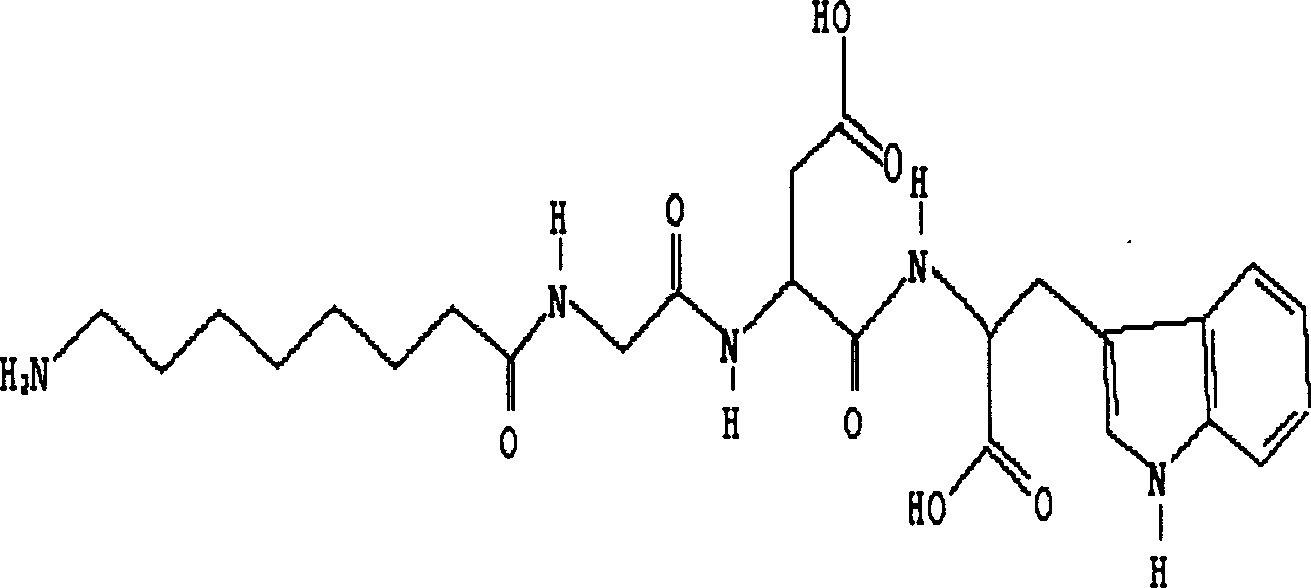
[0016] Example 1: H 2 N(CH 2 ) 7 The structure of -CO-Gly-Asp-Trp ( figure 1 ), its synthesis, purification, identification are as follows:
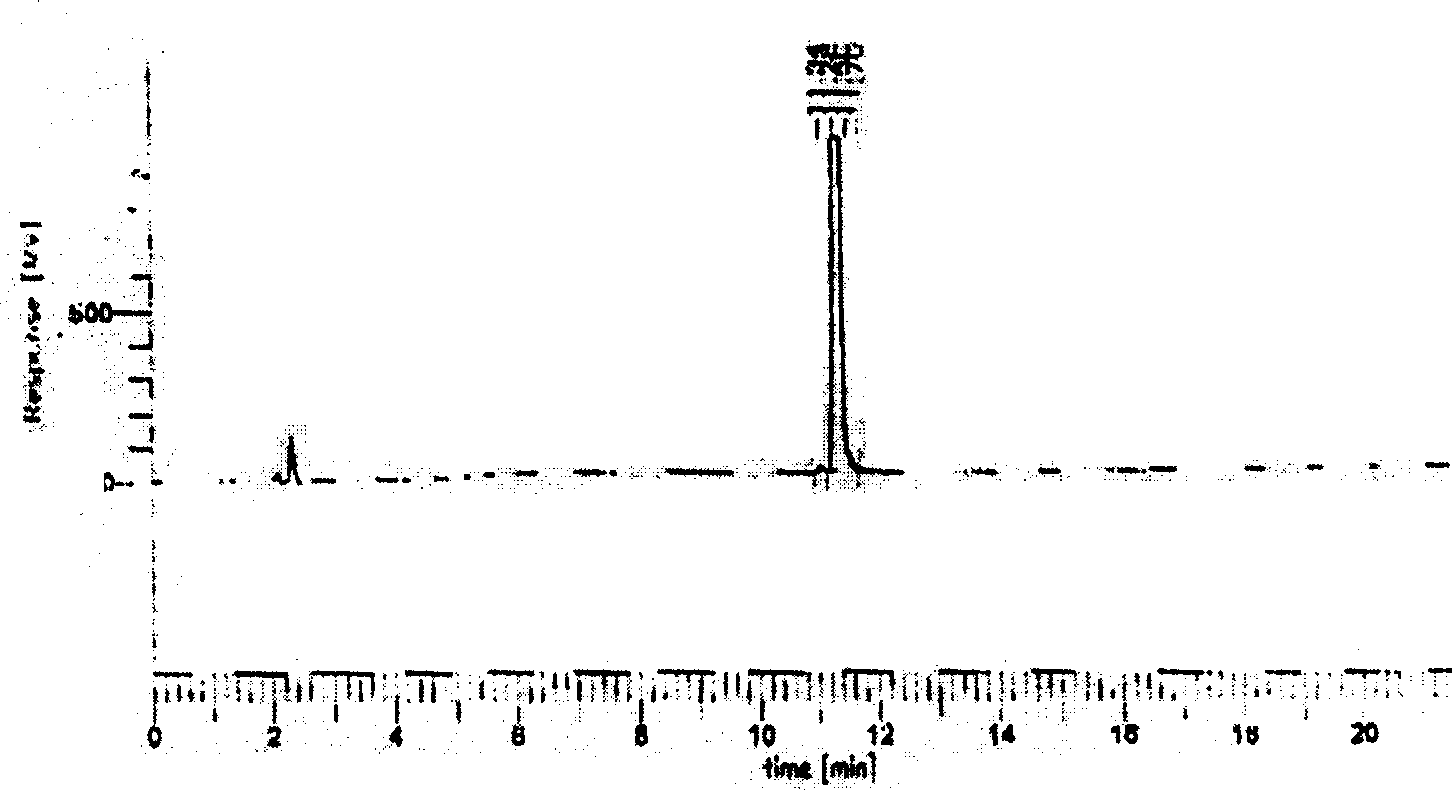
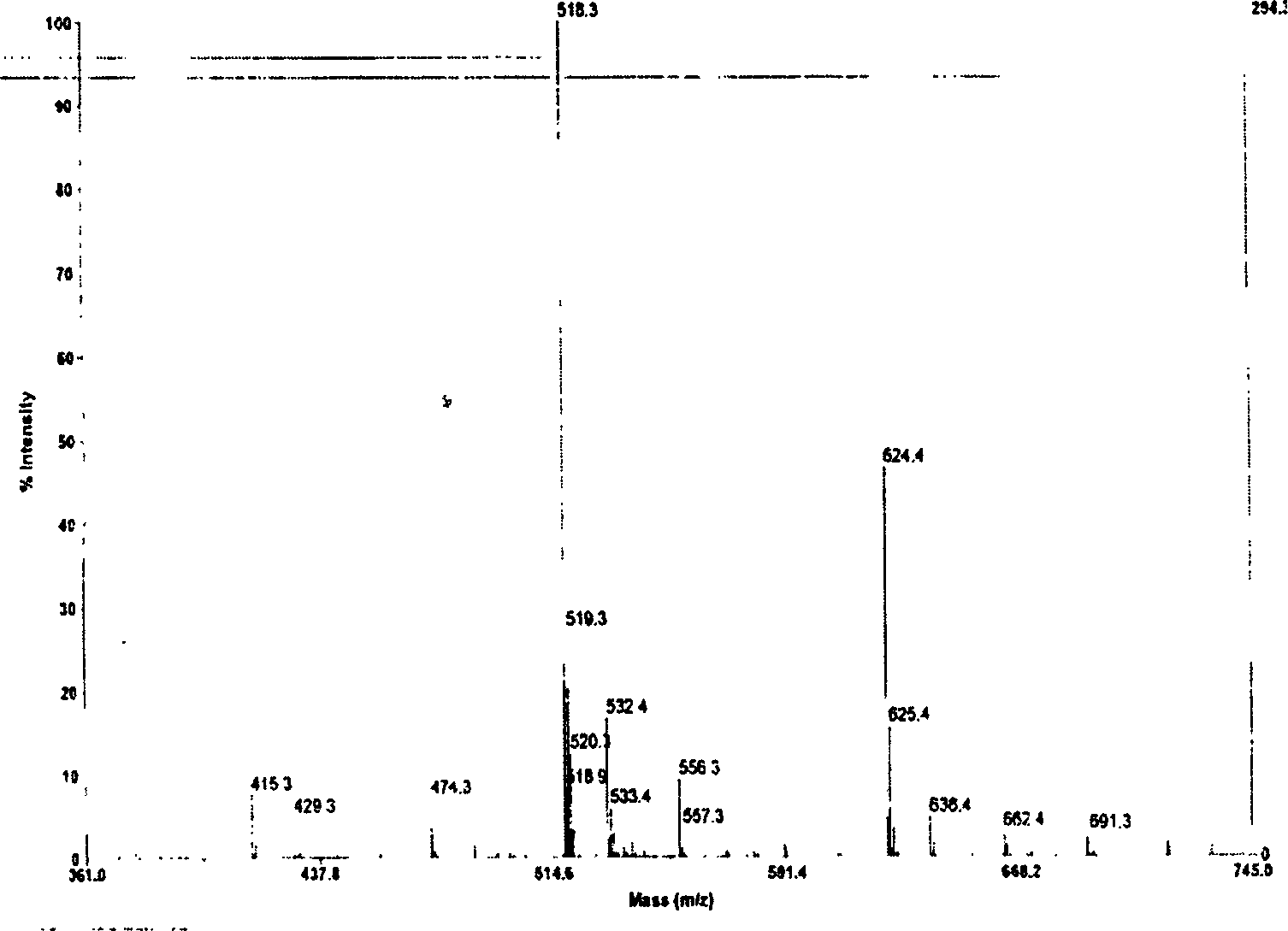
[0017] Using the 9-fluorenylmethyloxycarbonyl (Fmoc) protocol, Wang resin was used initially, and the peptide chain was extended from the C-terminal to the N-terminal according to the tetrapeptide sequence, and BOP / HOBt (1-hydroxyphenylacryltriazole) was added to each condensation step ) as a condensing agent, the Fmoc protecting group is removed with 20% hexahydropyridine in DMF (dimethylformamide) solution after condensation. After the peptide chain was synthesized, 95% TFA (trifluoroacetic acid) was used to stir at room temperature to cleave the peptide chain from the resin while removing the side chain protecting group, and the ninhydrin reagent was used to detect the completeness of deprotection and condensation. The synthetic peptide was purified by RP-HPLC (reverse-phase high-pressure liquid chromatography) C18 reverse-phase c...
Embodiment 2
[0018] Embodiment 2: Activity and stability experiment of antithrombotic effect
[0019] Blood was collected through the cubital vein on an empty stomach in the early morning, anticoagulated with 3.8% sodium citrate (the volume ratio of blood to anticoagulant was 9:1), centrifuged at 1000r / min for 8min at room temperature, and the upper plasma was taken as platelet-rich Plasma (PRP platelet-rich plasma), isolated PRP. The remaining blood was centrifuged at 3000r / min for 10min, and the upper plasma was collected to obtain platelet-poor plasma (PPP platelet-poor plasma). PRP was adjusted with PPP so that the number of platelets was about 250,000 / μL. Take 400 μL of PRP, add 0.9% NaCL (negative control) and different concentrations of RGDS (100, 80, 60, 30uM positive control) / small peptide (see Example 1) (10, 5, 2, 1uM final concentration) 50ul respectively, The platelet aggregation rate was measured by turbidimetry (the measuring instrument was Packs-4 platelet aggregation inst...
Embodiment 3
[0020] Embodiment 3: the specific research of antithrombotic effect
[0021] (1) Under aseptic conditions, take the umbilical cord of a fetus with normal delivery or caesarean section, squeeze out the blood in the umbilical cord vessel, put it into cold sterile D-hank's solution, and culture the umbilical cord vein endothelial cells within 3 hours. (2) Primary culture of human umbilical vein endothelial cells (HUVEC). (3) Cell de-adhesion experiment: HUVEC (7.5×10 4 / well) 1 mL was inoculated in a 24-well culture plate and cultured for 2 days to form a monolayer. Rinse twice with serum-free medium, discard the medium, add (see Example 1) (2.3, 5.5, 11, 22mM) 200uL into a 24-well culture plate, incubate together at 37°C for 4h, use serum-free The culture medium was rinsed 3 times to wash away the detached cells. (4) MTT method: Add 20uL of MTT to each well, and place the 24-well culture plate in CO 2 Incubator (37°C, 5% CO 2 ) for 4 h, discard the MTT supernatant, add 300 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com