Use of a VEGF antagonist in treating retinopathy of prematurity
A technology of retina and antagonists, applied in the direction of antibody medical ingredients, medical preparations containing active ingredients, anti-animal/human immunoglobulin, etc., can solve problems such as permanent damage to the peripheral retina
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Pharmacokinetic Model for Predicting Ocular and Systemic Exposure of Intravitreal Ranibizumab in Infants
[0082] To model ocular and systemic exposures of ranibizumab and bevacizumab in infants, two key relationships were established based on published data:
[0083] 1. Prediction of ocular clearance and vitreous concentration in relation to children's age and vitreous chamber depth and vitreous gel density;
[0084] 2. Prediction of the relationship between child age and weight for systemic concentrations and PK parameters for systemic deployment (allometric scaling).
[0085] Vitreous concentrations of ranibizumab and bevacizumab were calculated using vitreous volumes. This is calculated from the partial spherical volume with a height equal to the vitreous chamber depth (VCD) and a diameter equal to the axial length (AL) of the eye. Using linear regression models and published data (Fledelius (1992) Acta Ophthalmol Suppl 204:10-15), the association between VCD and ...
Embodiment 2
[0091] Determination of the ranibizumab dose for the treatment of infants with ROP
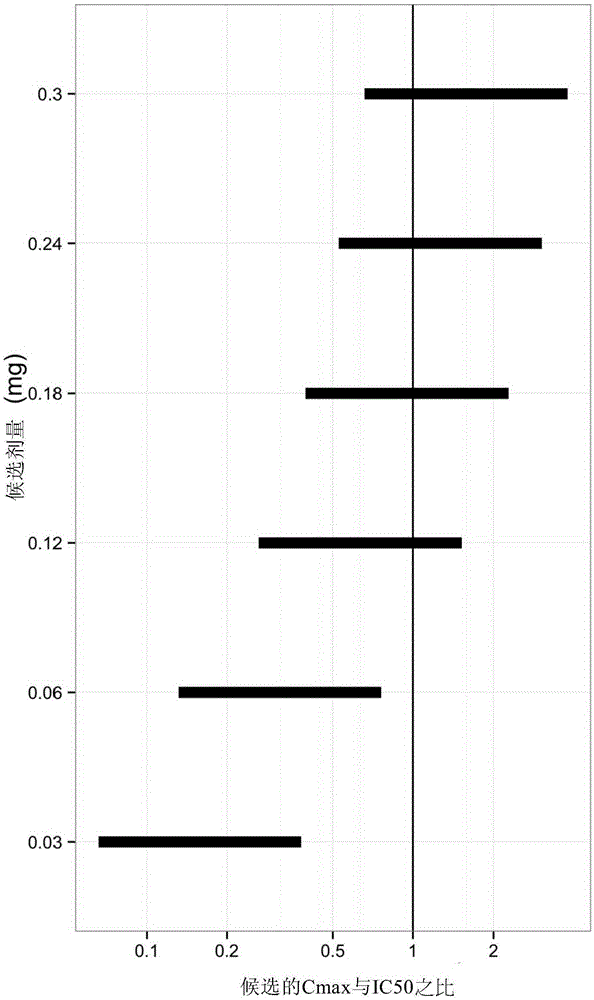
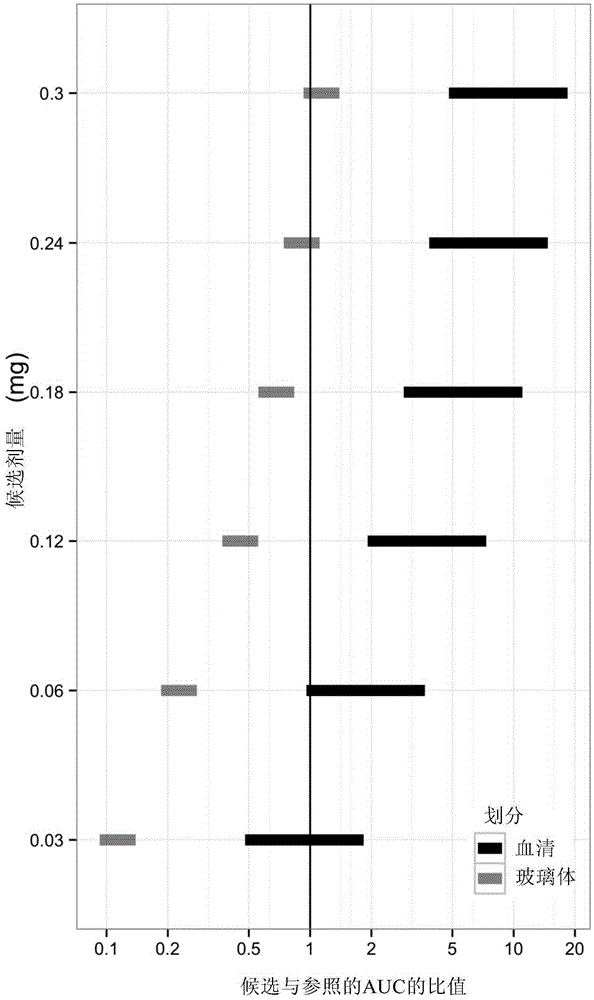
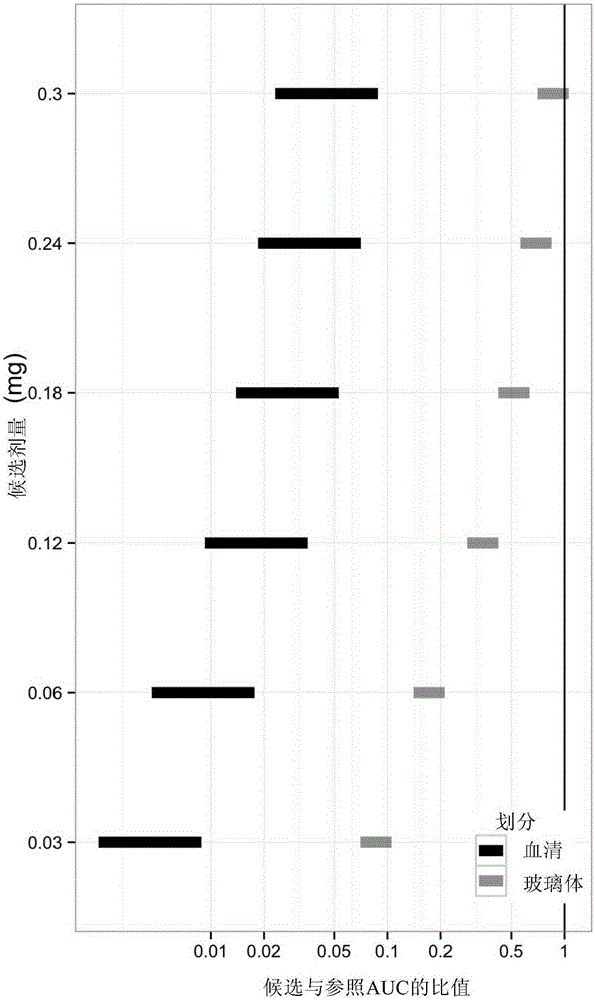
[0092] Using the pharmacokinetic model described in Example 1, predicted ocular and systemic exposures in infants receiving intravitreal ranibizumab were compared with exposures in adults receiving 0.5 mg ranibizumab intravitreal injection , because the efficacy and safety profile in adults at this dose level and dosing model is known.
[0093] For three different parameters: (i) maximum serum concentration (Cmax), which provides a measure of acute toxicity, (ii) serum area under the curve (AUC), which provides an indication of potential long-term toxicity associated with continuous inhibition of systemic VEGF Metrics, and (iii) Vitreous AUC, which provides a measure of efficacy correlated with continuous inhibition of ocular VEGF, to calculate the Ratio of Exposure to Ranibizumab.
[0094] The ratio of predicted exposure in infants to exposure in adults represents a measure of the likelihood...
Embodiment 3
[0100] Clinical study to determine the effect of two different doses of intravitreal ranibizumab in infants with ROP
[0101] The proposed study examines during a 16-week post-injection period: (i) whether ranibizumab provides similar therapeutic effects in ROP as reported in the BEAT-ROP study for bevacizumab; (ii) ranibizumab whether a lower dose of ranibizumab could achieve similar results in controlling ROP and (iii) whether the two doses of ranibizumab differed in their effect on systemic VEGF inhibition. This first phase is followed by a 5-year non-intervention period during which infants receiving treatment will undergo two long-term ophthalmic and pediatric developmental assessments (at 2 and 5 years), including VEGF-dependent organs such as the heart, lungs , vascular system and brain directional examination.
[0102] The study will enroll 40 patients who need treatment for zone I ROP (stage 1+, 2+, 3+ / - or AP-ROP) or central zone II ROP (stage 3+) according to the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com