Empagliflozin crystal forms, preparation methods and uses thereof, and pharmaceutical composition
A technology of empagliflozin and composition, which is applied in the direction of drug combination, preparation of sugar derivatives, chemical instruments and methods, etc., can solve the problem of unfavorable crystal form stability preparation storage stability, affecting preparation operability, crystal form A Low purity and other issues, to achieve the effect of improving bioavailability, reducing sieving time, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0075] Preparation Example 1 Preparation of empagliflozin
[0076] Empagliflozin was prepared by referring to the preparation method of the examples of the patent document WO2006 / 117359A1. Specifically: take 2.85g of (R)-3-(4-methylphenylsulfonyloxy)-tetrahydrofuran and add 3.00g of 1-chloro-4-(β-D-glucopyranose-1-yl )-2-(4-Hydroxybenzyl)-benzene and 4.36 g of cesium carbonate in a mixture of 38 mL of dimethylformamide. The mixture was stirred at 75° C. for 5 hours, then an additional 4.40 g of cesium carbonate and 2.87 g of (R)-3-(4-methylphenylsulfonyloxy)-tetrahydrofuran were added. After stirring for another 16 hours at 75°C, the mixture was cooled to room temperature and brine was added. The resulting mixture was extracted with ethyl acetate, and the combined organic extracts were dried over anhydrous sodium sulfate. The residue was purified by column (dichloromethane / methanol 1:0→5:1). Yield is 2.1 g, yield 57%. Mass spectrometry (ESI + ): m / z=451 / 453 (Cl)[M+H] ...
preparation example 2
[0077] Preparation example 2 Preparation of Crystal Form A of Empagliflozin
[0078] The crystal form A of empagliflozin was prepared by referring to the preparation method of the example of patent document WO2006 / 117359A1. Specifically: 2.1 g of empagliflozin was dissolved in 50 mL of ethyl acetate (containing 0.5-3% water) heated to 50°C. The solution was cooled slowly (about 1-4 hours) to room temperature. After 72 hours, it was filtered and rinsed twice with ethyl acetate (containing 0.5-3% water) to obtain a white solid, which was dried overnight at 45° C., and its XRPD pattern was as follows: Figure 5 As shown, it is consistent with the crystal form A disclosed in the patent document WO2006 / 117359A1.
Embodiment 1
[0080] Get 29.01mg of the crystal form A of empagliflozin prepared in Preparation Example 2 in a 20mL glass vial, add 5.8mL of ethyl acetate, and stir for 3 hours in a water bath at 80°C, and the system basically dissolves (the empagliflozin in the solution The dosage of crystal form A is 1 time of its solubility in ethyl acetate at 80°C), and after hot filtration, the filtrate is directly placed at 4°C and stirred, and a white solid is precipitated in 2 hours; after centrifugation, empagliflozin is obtained. Form I, the yield is 24.26mg, the yield is 76%.
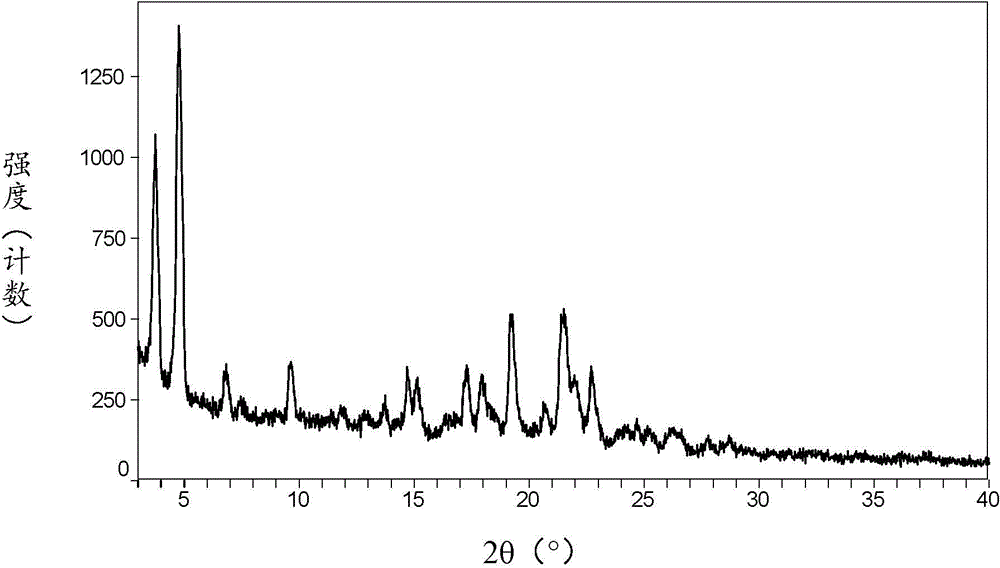
[0081] Its XRPD pattern is as follows figure 1 Shown is the crystal form I of empagliflozin.
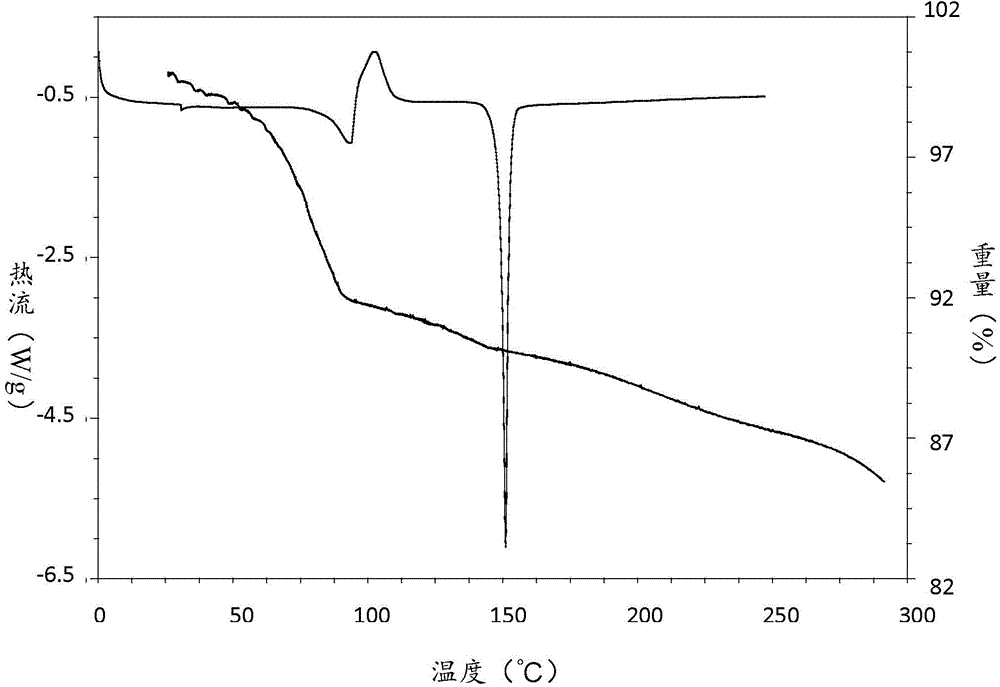
[0082] Its TGA / DSC spectrum is as follows figure 2 As shown, the melting point is 149°C, the weight loss is 1.1% before 50°C, and the weight loss is 8.8% at 50-150°C, which is about one molecule of empagliflozin combined with 0.5 ethyl acetate molecules.
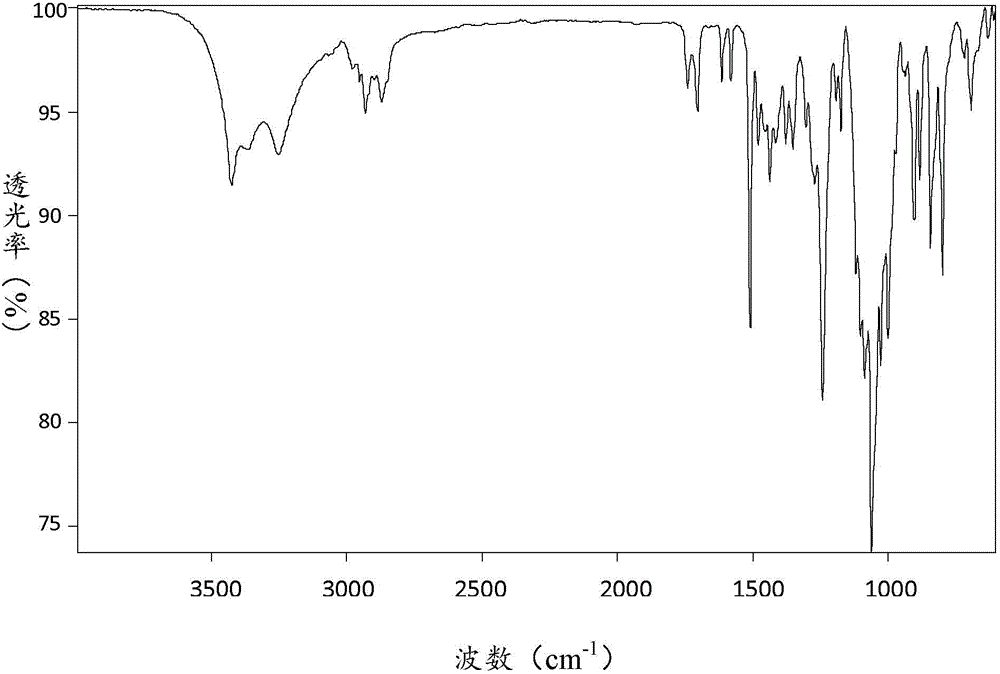
[0083] Its FT-IR spectrum is as image 3 Shown, compared with the FT-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com