Liraglutide composition and application thereof
A technology of liraglutide and polypeptide composition, which can be applied in the directions of drug combination, peptide/protein component, anhydride/acid/halide active ingredient, etc., and can solve the problems of inconvenient administration and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
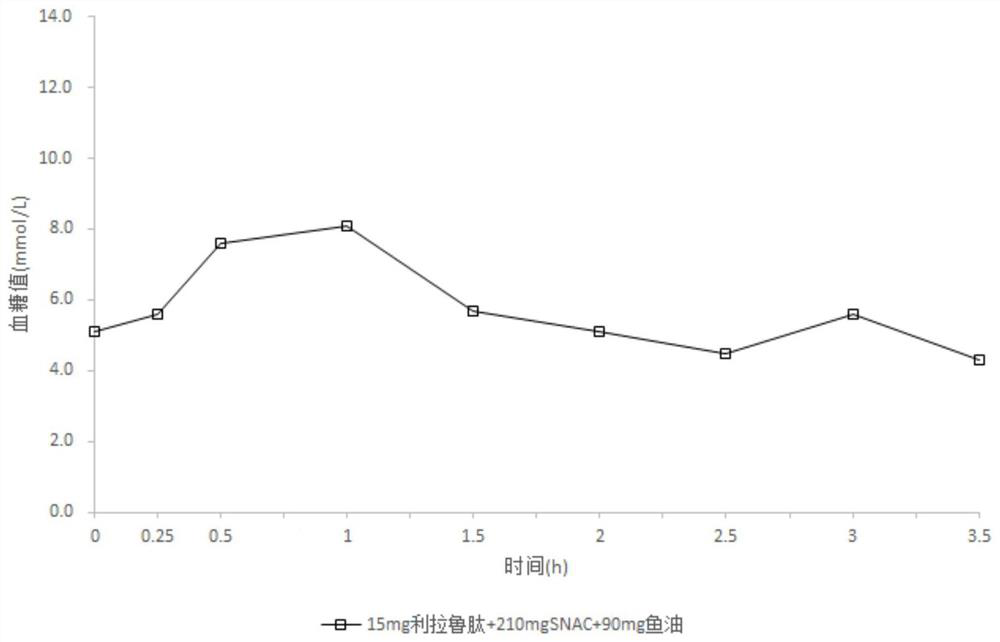
[0072] Composition A
[0073] Liraglutide 15mg Sodium N-(8-(2-hydroxybenzoyl)amino)octanoate 210mg fish oil 90mg
[0074] Preparation
[0075] Mix 15mg of liraglutide, 210mg of sodium N-(8-(2-hydroxybenzoyl)amino) octanoate, and 90mg of fish oil, put it into a capsule, and coat it. Weigh Eudragit L30D, talcum powder, and polyethylene glycol, dissolve with dichloromethane and isopropanol to obtain a coating solution, and spray the coating solution on the surface of the capsule to obtain an enteric-coated capsule. The flow rate of the coating solution is 0.5ml / min, the coating temperature is 30°C-40°C, and the coating weight gain is 7%.
Embodiment 2
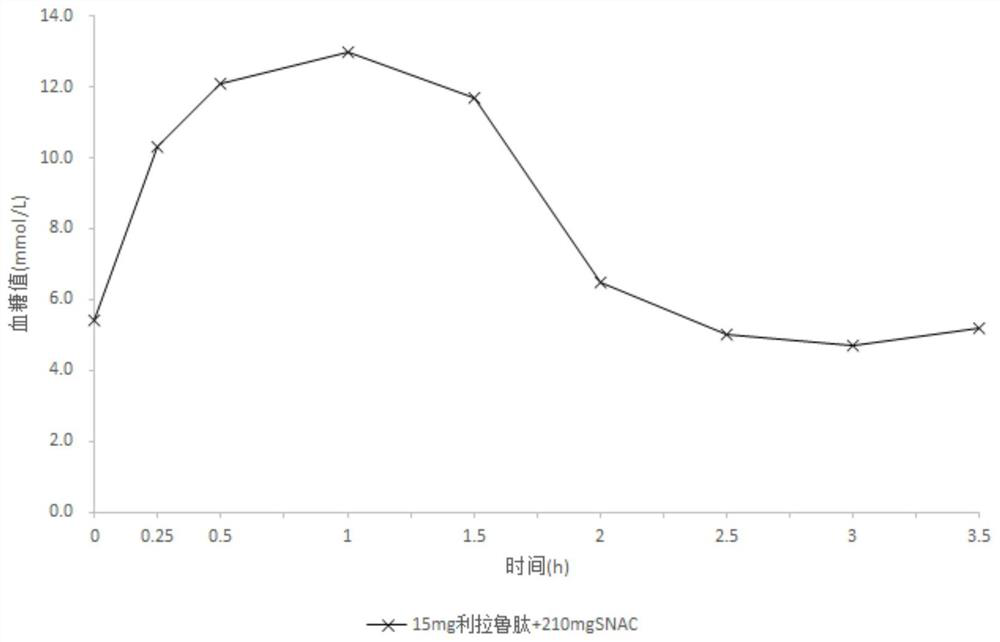
[0077] Composition B
[0078] Liraglutide 15mg Sodium N-(8-(2-hydroxybenzoyl)amino)octanoate 210mg fish oil 0mg
[0079] Preparation
[0080] Mix 15 mg of liraglutide and 210 mg of sodium N-(8-(2-hydroxybenzoyl)amino) caprylate, put it into a capsule, and coat it. Weigh Eudragit L30D, talcum powder, and polyethylene glycol, dissolve with dichloromethane and isopropanol to obtain a coating solution, and spray the coating solution on the surface of the capsule to obtain an enteric-coated capsule. The flow rate of the coating solution is 0.5ml / min, the coating temperature is 30°C-40°C, and the coating weight gain is 7%.
Embodiment 3
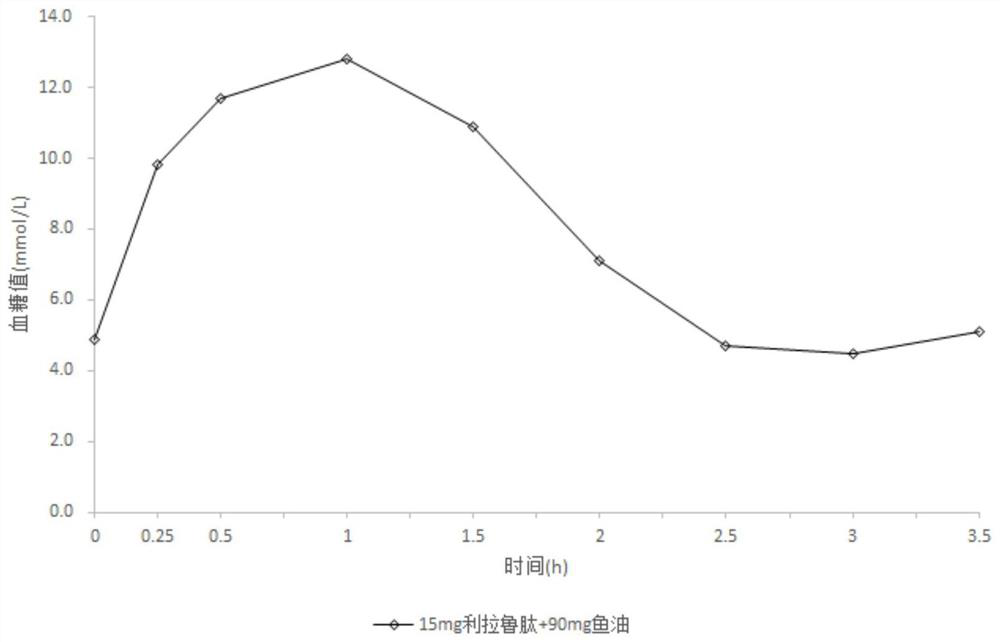
[0082] Composition C
[0083] Liraglutide 15mg Sodium N-(8-(2-hydroxybenzoyl)amino)octanoate 0mg fish oil 90mg
[0084] Preparation
[0085] Mix 15mg liraglutide and 90mg fish oil, put it into a capsule, and coat it. Weigh Eudragit L30D, talcum powder, and polyethylene glycol, dissolve with dichloromethane and isopropanol to obtain a coating solution, and spray the coating solution on the surface of the capsule to obtain an enteric-coated capsule. The flow rate of the coating solution is 0.5ml / min, the coating temperature is 30°C-40°C, and the coating weight gain is 7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com