A composition for lightening skin and hair
A technology of composition and mixture, which is applied in skin care preparations, skin diseases, drug combinations, etc., and can solve problems such as high sensitization potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0273] Pigmentation effects on melanoma cell cultures
[0274] Take 7.5×10 3 Concentration of cells / well B16V mouse melanoma cells were dispersed in 96-well microtiter plates. in RPMI medium at 37 °C and 5% CO 2 Cultured in medium for 24 hours, enriched with 10% fetal bovine serum, added various concentrations of test substances and 0.3 mM tyrosine and 10 nM α-MSH (α-melanostimulating hormone) and continued to culture for 96 hours. The maximum concentration of the test substance used corresponds to the IC of the cytotoxicity test 20 0.1 times the value. Only tyrosine and α-MSH were added to the control. After the cultivation, a sodium hydroxide solution (final concentration: 1M) was added to the culture medium and absorbance at 400 nm was measured 3 hours later (A).
[0275] Inhibition of pigmentation in the presence of test compounds was calculated using the following equation:
[0276] Pigmentation inhibition rate (%)=100-[(A 测试化合物 / A 对比样 )×100]
[0277] in
[0278...
Embodiment 2
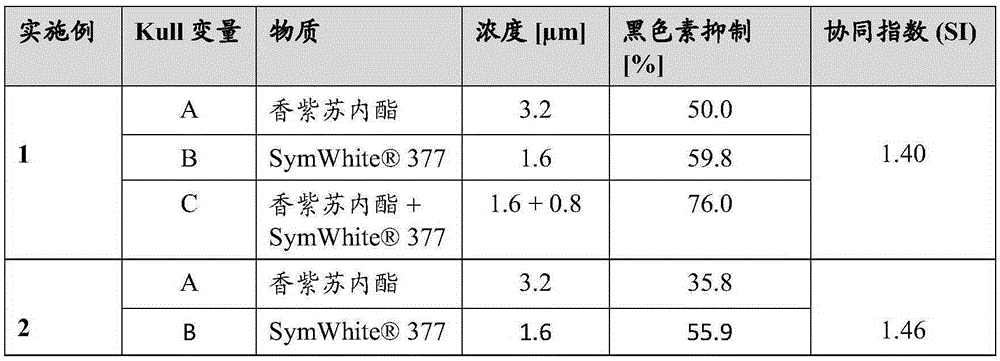
[0285] Synergistic effect of melanin inhibition in vitro
[0286] The experimental process is described in Example 1. The results are shown in Table 2:
[0287] Table 2
[0288] melanin suppression
[0289]
[0290]
[0291] Synergy Index (SI) 1 Calculated according to the following Kull equation:
[0292] S I = C * D A + C * E B
[0293] in
[0294] A: Individual results for substance A
[0295] B: Individual results for substance B
[0296] C: Results of a mixture of A and B
[0297] D: Percentage of substance A in the mixture
[0298] E: Percentage of substance B in the mixture
[0299] Compared with the examples given in the literature, where A, B and C decreased values (i.e. the minimum concentration of inhibition in the antimicrobial test) mean be...
Embodiment 3
[0302] In Vitro Skin Pigmentation
[0303] Pigmented pigskin was excised from animals (slaughtered for meat, as described in EP193927 for pigskin samples including sub-epidermal fat layer) and cut into 4x4x3mm pieces (lengthxwidthxthickness) and placed in soaking Air-liquid interface cultures on sterile cotton pads with 5 ml of custom-made DMEM (Dulbecco's Modified Eagle Medium). Sample at 37°C, 5% CO 2 Tested after 24 hours of acclimatization. O / W emulsions without (= control) and with test compound (described in more detail below) were applied topically And cultivated for 6 days. Tissue sections were prepared and pellets were melanin stained by the Fontana-Masson technique. Particles were quantified by image analysis. The results are shown in Tables 3.1 and 3.2.
[0304] chart 3.1
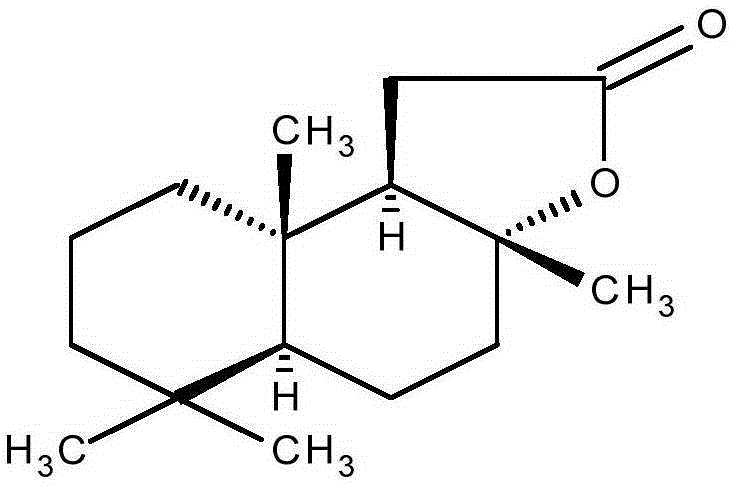
[0305] Pigmentation of sclareolide on skin in vitro
[0306] test substance
Dosage weight%
Melanin score compared to control
Sclareolactone
0.1%
-42%
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com