3,4-perylene dicarboxylate compound, and synthetic method and applications thereof
A technology of ester compound and perylene dicarboxylic acid, which is applied in the field of 3,4-perylene dicarboxylate ester compound and its synthesis, can solve the problems of not being seen and few positions for continuing reaction, and achieve high selectivity, The effect of simple reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Dissolve 600 mg of n-butyl perylene-3,4-anhydride-9,10-perylenedicarboxylate in 30 ml of freshly distilled quinoline, add 500 mg of Cu 2 O, under anaerobic conditions, reflux reaction for 48 hours, pour the reacted solution into 100 milliliters of 0.1 mol / liter sulfuric acid aqueous solution to form a precipitate, suction filter, wash and dry to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane. Product A was obtained in 367 mg, yield 61%.
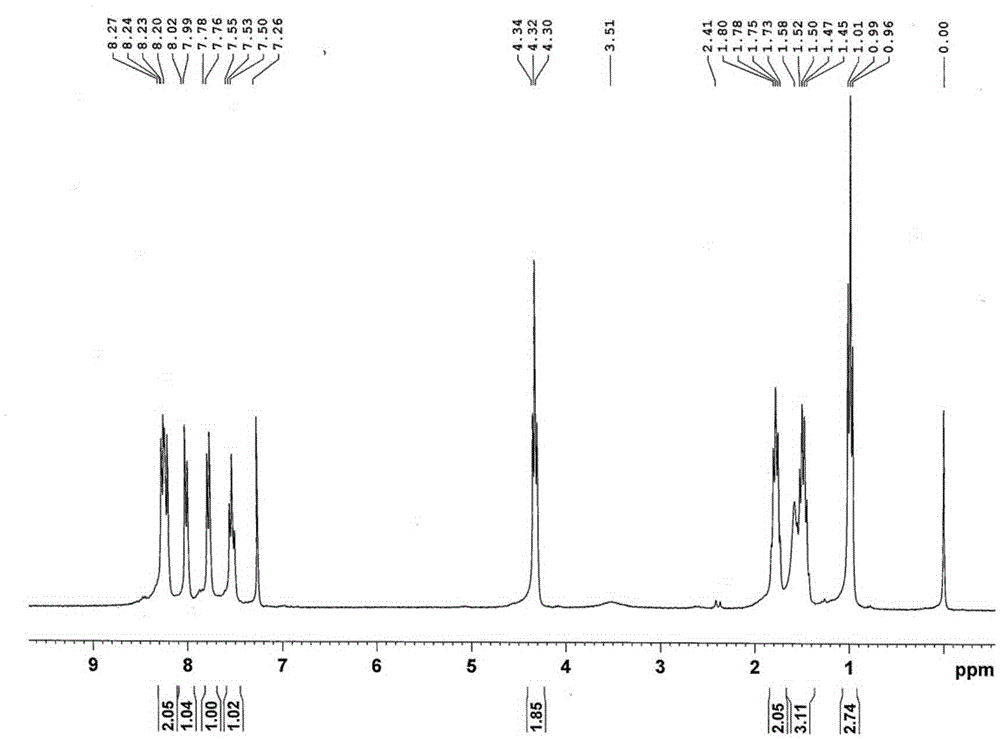
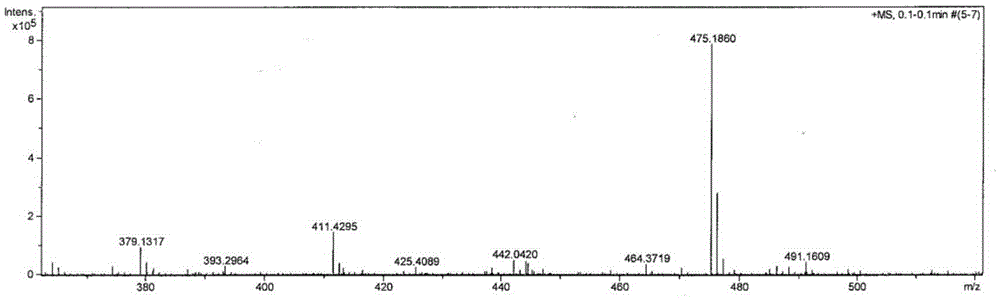
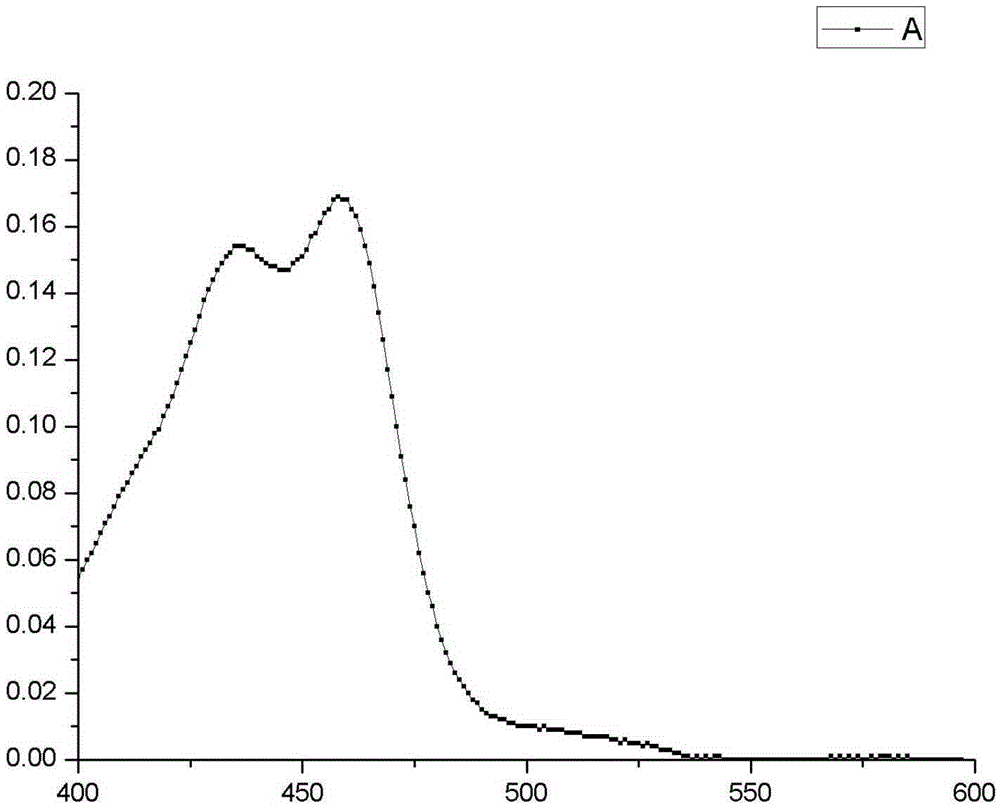
[0037] The structural characterization of product A is as follows Figure 1-2 as shown,
[0038] The hydrogen spectrum is divided into three parts: chemical shift from 1 to 2, here is the hydrogen on the alkyl chain on the ester group in the 3,4-perylene dicarboxylate compound, from left to right is β, γ , δ hydrogen; 4 to 5 are the α hydrogen on the alkyl chain on the ester group; there are four groups of peaks between 7 and 9, of which 7.26 is CDCl 3...
Embodiment 2
[0041] Dissolve 600 mg of n-propyl perylene-3,4-anhydride-9,10-perylenedicarboxylate in 20 mL of freshly distilled quinoline, add 300 mg of Cu 2 O, under anaerobic conditions, reflux reaction for 40 hours, pour the reacted solution into 80 ml of 0.05 mol / L hydrochloric acid aqueous solution to form a precipitate, suction filter, wash, and dry to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane. 378 mg of product B were obtained, yield 63%.
Embodiment 3
[0043] Dissolve 600 mg of n-pentyl perylene-3,4-anhydride-9,10-perylenedicarboxylate in 25 ml of freshly distilled quinoline, add 600 mg of Cu 2 O, under anaerobic conditions, reflux reaction for 52 hours, pour the reacted solution into 90 milliliters of 0.08 mol / liter nitric acid aqueous solution to form a precipitate, suction filter, wash and dry to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane. 396 mg of product C were obtained, yield 66%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com