Antibody-SN-38 immunoconjugates with a CL2A linker
A CL2A-SN-38, -PABO-CO-20-O-SN-38 technology, applied in anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, Antibodies, etc., can solve the problems of reducing the therapeutic window, non-existent therapeutic efficacy, discontinuity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
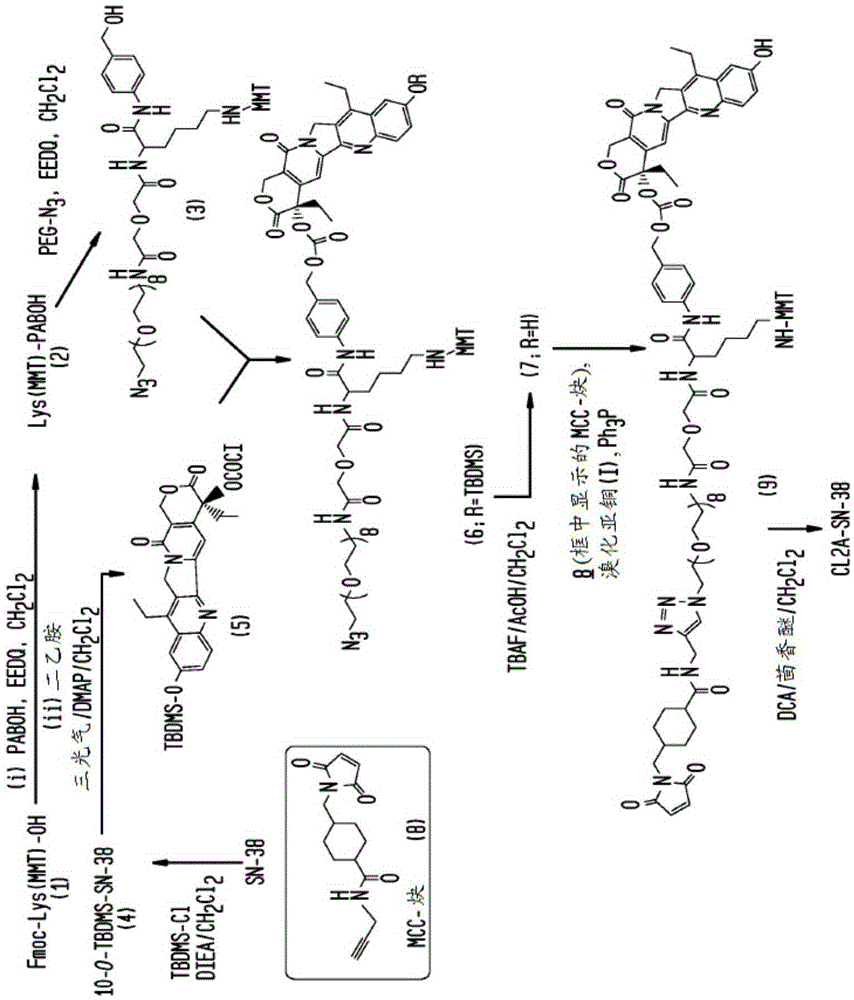
[0402] Example 1: Preparation of CL2A-SN-38
[0403] A preferred reaction scheme for the synthesis of CL2A-SN-38 is shown in figure 1 , including the following steps for an improved method for large-scale production.
[0404] O-(2-azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl base)-Lys(MMT)-PABOH]heptaethylene glycol (intermediate 3, figure 1 ) preparation: In a 500-mL single-necked flask, add commercially available Fmoc-Lys(MMT)-OH (16 g), p-aminobenzyl alcohol (3.26 g), and EEDQ (6.52 g), followed by anhydrous dichloromethane (80mL). After stirring overnight, diethylamine (25 mL) was added and after a further 6 hours the reaction mixture was concentrated to a volume of ~50 mL. It was diluted with heptane, and the solution was concentrated back to 50 mL. Two additional chases with heptane (50 mL each) provided a biphasic mixture with a gummy material at the bottom. The gummy material was dissolved in dichloromethane (24 mL), stirred, and worked up to slowly add heptane ...
Embodiment 2
[0408] Example 2. Conjugation of CL2A-SN-38 to antibodies
[0409] Anti-CEACAM5 humanized MAb, hMN-14, anti-CD22 humanized MAb, hLL2, anti-CD20 humanized MAb, hA20, anti-EGP-1 humanized MAb, hRS7 and anti-mucin humanized MAb The derivatized MAb, hPAM4, was used in these studies. Gently reduce each antibody with tris(2-carboxyethyl)phosphine (TCEP) in phosphate buffered saline at a pH in the range of 7-7.4, adjust the pH to 6.5, and use 5-10% v / v was reacted with DMSO as a co-solvent with ~10-fold molar excess of CL2A-SN-38 and incubated for 20 min at ambient temperature. A 10-fold molar excess of N-ethylmaleimide relative to the antibody was used as an aqueous solution to block any excess thiols.
[0410] The conjugate was purified by tangential flow filtration (TFF) using 20-30 diafiltration volumes of final formulation buffer 25 mMMES, pH 6.5. This method avoids tedious serial purifications on size-exclusion and hydrophobic columns, thus enabling the purification of hund...
Embodiment 3
[0413] Example 3. In vivo therapeutic efficacy in preclinical models of human pancreatic or colon cancer
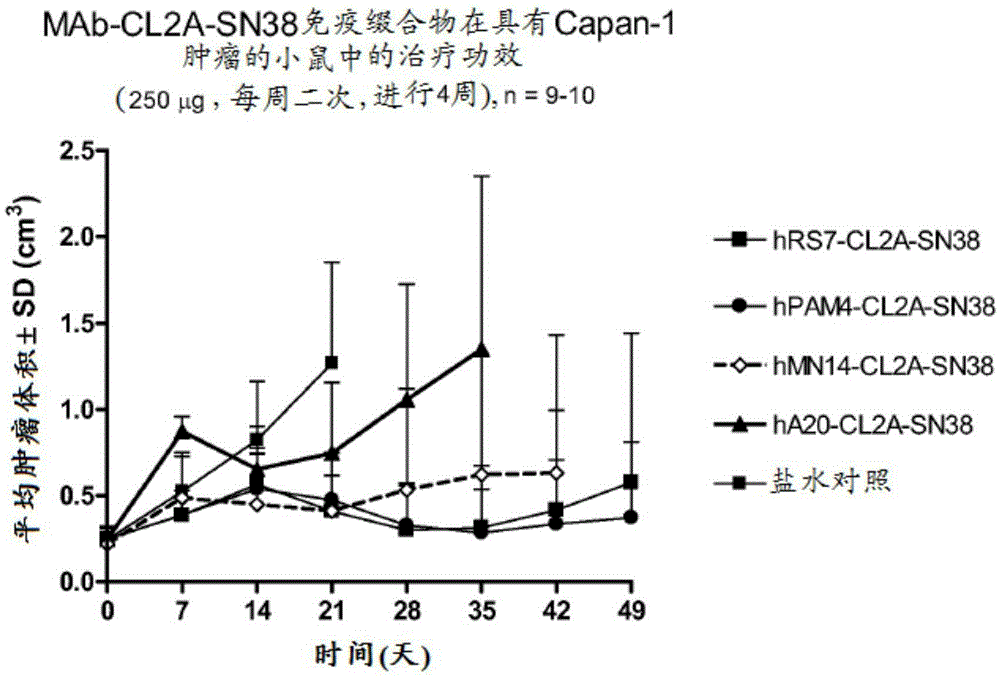
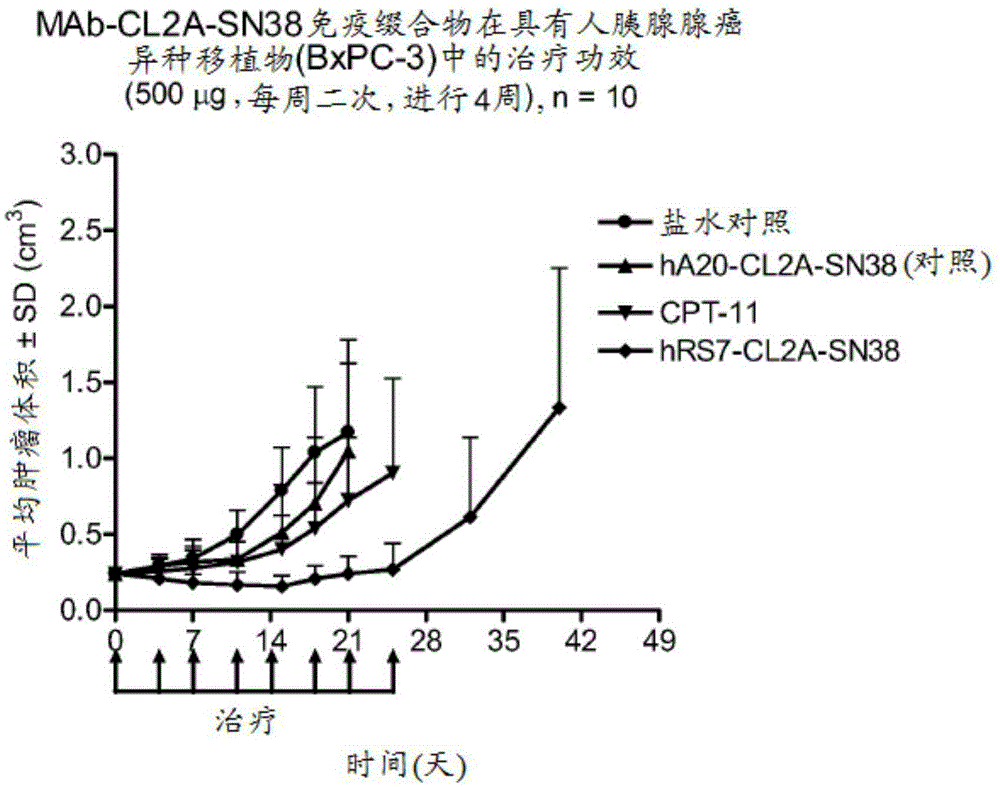
[0414]Immunocompromised athymic female nude mice bearing subcutaneous human pancreatic or colon tumor xenografts were treated or left untreated with specific CL2A-SN-38 conjugates or control conjugates. The therapeutic efficacy of the specific conjugates was observed. figure 2 shows the Capan1 pancreatic tumor model in which specific CL2A-SN-38 conjugates of the hRS7 (anti-EGP-1), hPAM4 (anti-mucin) and hMN-14 (anti-CEACAM5) antibodies showed higher - Better efficacy of SN-38 conjugate (anti-CD20) and untreated control. Similarly, in the BXPC3 model of human pancreatic cancer, specific hRS7-CL2A-SN-38 showed better therapeutic efficacy than control treatment ( image 3 ). Likewise, treatment with specific hMN-14-CL2A-SN-38 was more effective than non-treatment in an aggressive LS174T model of human colon cancer ( Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com