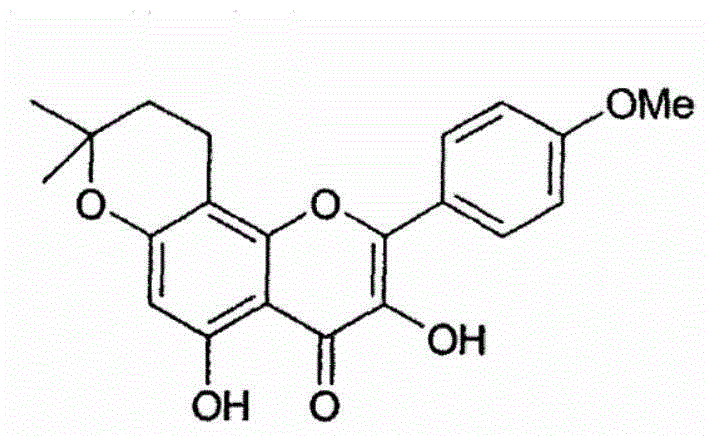
Anhydroicaritin oral preparation and preparation method thereof
A technology for oral preparations of icariin, which is applied in the field of oral preparations of icariin and its preparation, can solve the problems of complicated process, potential safety hazards, and limited improvement, and achieve the effect of simple preparation process and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Icariin 1g
[0020] Diethylene glycol monoethyl ether 20g
[0021] Povidone 5g
[0022] Preparation Process:
[0023] The prescribed amount of icariin and povidone is weighed, added to the prescribed amount of diethylene glycol monoethyl ether, stirred to dissolve, and then the solution is filtered and then divided into oral liquid bottles.
Embodiment 2
[0025] Icariin 1g
[0026] Diethylene glycol monoethyl ether 60g
[0027] Povidone 15g
[0028] Preparation Process:
[0029] The prescribed amount of icariin and povidone is weighed, added to the prescribed amount of diethylene glycol monoethyl ether, stirred to dissolve, and then the solution is filtered and then divided into oral liquid bottles.
Embodiment 3
[0031] Icariin 1g
[0032] Diethylene glycol monoethyl ether 40g
[0033] Povidone 10g
[0034] Preparation Process:
[0035] The prescribed amount of icariin and povidone is weighed, added to the prescribed amount of diethylene glycol monoethyl ether, stirred to dissolve, and then the solution is filtered and then divided into oral liquid bottles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com