Preparation method of 1,1-diphenyl ethane
A technology of diphenylethane and dichloroethane, which is applied in the field of preparation of 1,1-diphenylethane, can solve the problems of process pollution, catalyst can not be reused, low yield, etc., and achieve low process pollution , Environmentally friendly catalytic process, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
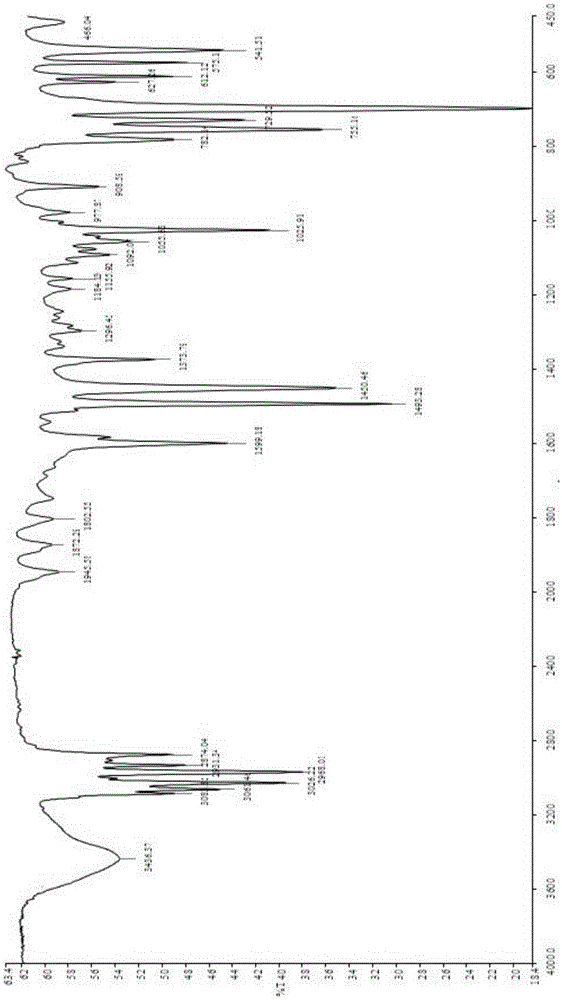
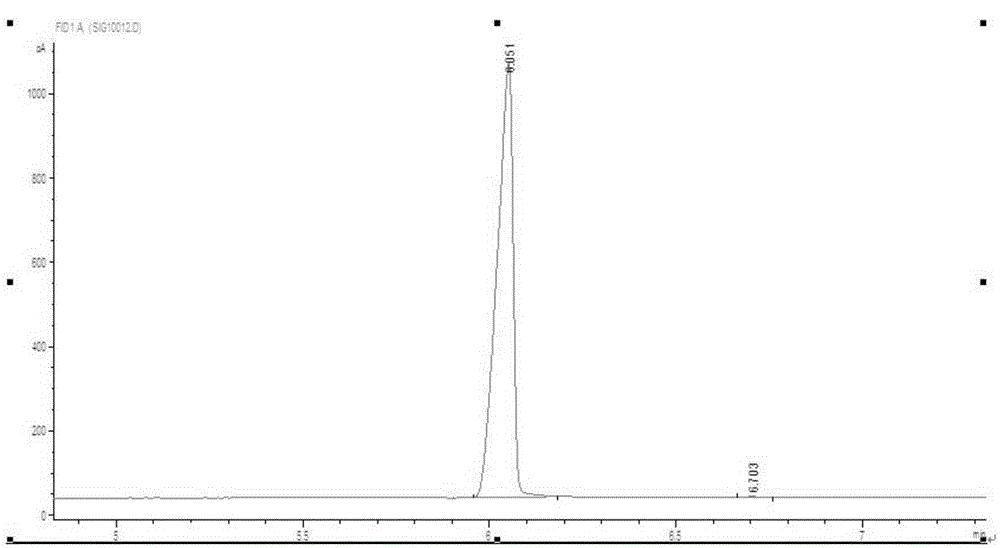
Image
Examples
Embodiment 1
[0027] With benzene 281.2g (3.6mol), ionic liquid catalyst triethylamine hydrochloride-aluminum trichloride [Et 3 NHCl-AlCl 3 ] 10g was added to the reaction bottle, and 59.4g (0.6mol) of 1,1-dichloroethane was added dropwise to the reaction system at a uniform speed at 40°C, and the drop was completed in 3 hours, and then the temperature was raised to 50°C for 1.5 hours.
[0028] After the reaction is finished, the temperature of the reaction system is first lowered to room temperature, then the reaction solution is allowed to stand for stratification, and the upper organic phase is separated, and the organic phase is washed with water and alkali to neutrality, and then the excess benzene is recovered by distillation to obtain 1, The crude product of 1-diphenylethane is collected by rectification at 126-129℃ / 8mmHg to obtain 1,1-diphenylethane.
Embodiment 2
[0030] With benzene 37.5g, ionic liquid catalyst triethylamine hydrochloride-zinc chloride [EtN 3 HCl-ZnCl 2 ] 10g was added to the reaction bottle, and 2.5g of 1,1-dichloroethane was added dropwise to the reaction system at a uniform speed at 80°C, and the drop was completed in 2 hours, and continued for 1 hour.
[0031] After the reaction is finished, the temperature of the reaction system is first lowered to room temperature, then the reaction solution is allowed to stand for stratification, and the upper organic phase is separated, and the organic phase is washed with water and alkali to neutrality, and then the excess benzene is recovered by distillation to obtain 1, The crude product of 1-diphenylethane is collected by rectification at 126-129℃ / 8mmHg to obtain 1,1-diphenylethane.
Embodiment 3
[0033] With benzene 98g, ionic liquid catalyst chloride 1-butyl-3-methylimidazole-aluminum trichloride [BMIMCl-AlCl 3 ] 2g was added to the reaction bottle, and 1,1-dichloroethane 100g was added dropwise to the reaction system at a uniform speed at 0°C, and the drop was completed in 4 hours, and continued for 2 hours.
[0034] After the reaction is finished, the temperature of the reaction system is first lowered to room temperature, then the reaction solution is allowed to stand for stratification, and the upper organic phase is separated, and the organic phase is washed with water and alkali to neutrality, and then the excess benzene is recovered by distillation to obtain 1, The crude product of 1-diphenylethane is collected by rectification at 126-129℃ / 8mmHg to obtain 1,1-diphenylethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com