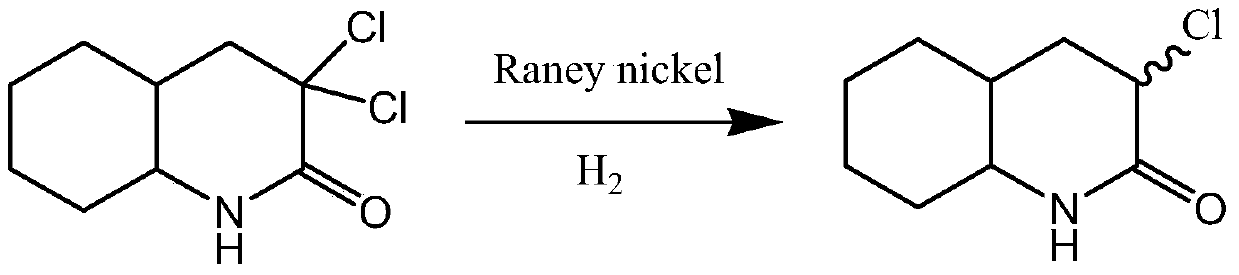
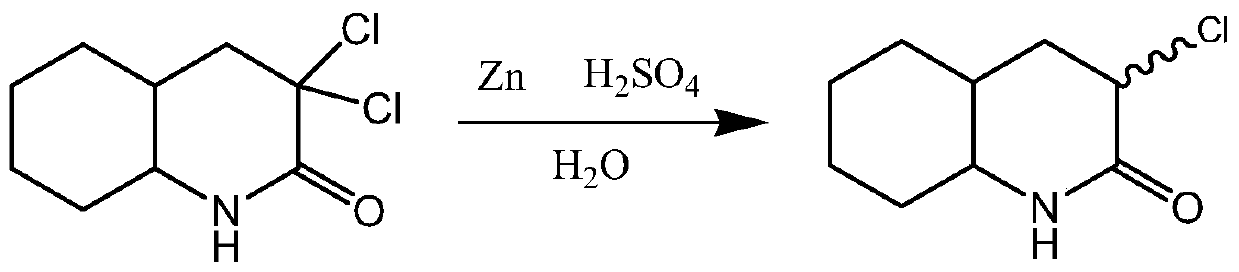
Synthetic process of 3-chloro-octahydro-2(1h)-quinolinone
A synthesis process, quinolinone technology, applied in the direction of organic chemistry, can solve problems such as poor selectivity, low conversion rate, and difficulty in achieving small test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 3,3-dichloro-octahydro-2(1H)-quinolinone 100g, 2kg of chloroform, 1kg of ethanol, 700g of 10% sulfuric acid in a 5L round bottom reaction flask equipped with a stirrer and a thermometer, Slowly add 60g of zinc powder under the condition of stirring at 20-25°C for about 3 hours, continue to stir for 1 hour and then stand for stratification. After the obtained organic layer is concentrated, add an equal proportion of ethanol / isopropyl ether to the residue of the kettle Mixed solvent 300ml, the precipitated solid product 3-chloro-octahydro-2(1H)-quinolinone was filtered, washed and dried to obtain off-white solid 3-chloro-octahydro-2(1H)-quinoline Ketones 75.3g, 1 H-NMR (CDCl 3 ), δ: 1.00~2.78(m, 12H), δ: 2.83~2.92(t, 1H), δ: 4.35(s, 1H).
[0028] Through gas chromatography analysis, its area-normalized content was 97.7%, and the yield was 87.1%.
Embodiment 2
[0030] Add 3,3-dichloro-octahydro-2(1H)-quinolinone 100g, 2kg of chloroform, 1kg of ethanol, 500g of 10% sulfuric acid in a 5L round bottom reaction flask equipped with a stirrer and a thermometer, Slowly add 60g of zinc powder under the condition of stirring at 20-25°C for about 3 hours, continue to stir for 1 hour and then stand for stratification. After evaporating the solvent, add an equal proportion of ethanol / isopropyl ether mixed solvent to the obtained organic layer. 300ml, the precipitated solid product was filtered, washed and dried to obtain 80.2g of off-white solid 3-chloro-octahydro-2(1H)-quinolinone, 1 H-NMR (CDCl 3 ), δ: 1.00~2.78(m, 12H), δ: 2.83~2.92(t, 1H), δ: 4.35(s, 1H). After gas chromatography analysis, the area normalized content was 85.8%, and the yield was 81.4%.
Embodiment 3
[0032] Add 100 g of 3,3-dichloro-octahydro-2(1H)-quinolinone, 2 kg of dichloromethane, 1 kg of methanol, and 10% sulfuric acid into a 5 L round-bottomed reaction flask equipped with a stirrer and a thermometer. 500g, add 55g of zinc powder slowly and gradually in about 3 hours at 20-28°C under stirring conditions, continue to stir for 1h and then stand for stratification. After evaporating the solvent, add an equal proportion of ethanol / isopropyl ether to the obtained organic layer Mixed solvent 300ml, the precipitated solid product was filtered, washed, and dried to obtain 76.1 g of off-white solid 3-chloro-octahydro-2(1H)-quinolinone, 1 H-NMR (CDCl 3 ), δ: 1.00~2.78(m, 12H), δ: 2.83~2.92(t, 1H), δ: 4.35(s, 1H). Through gas chromatography analysis, the area normalized content was 70.2%, and the yield was 63.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com