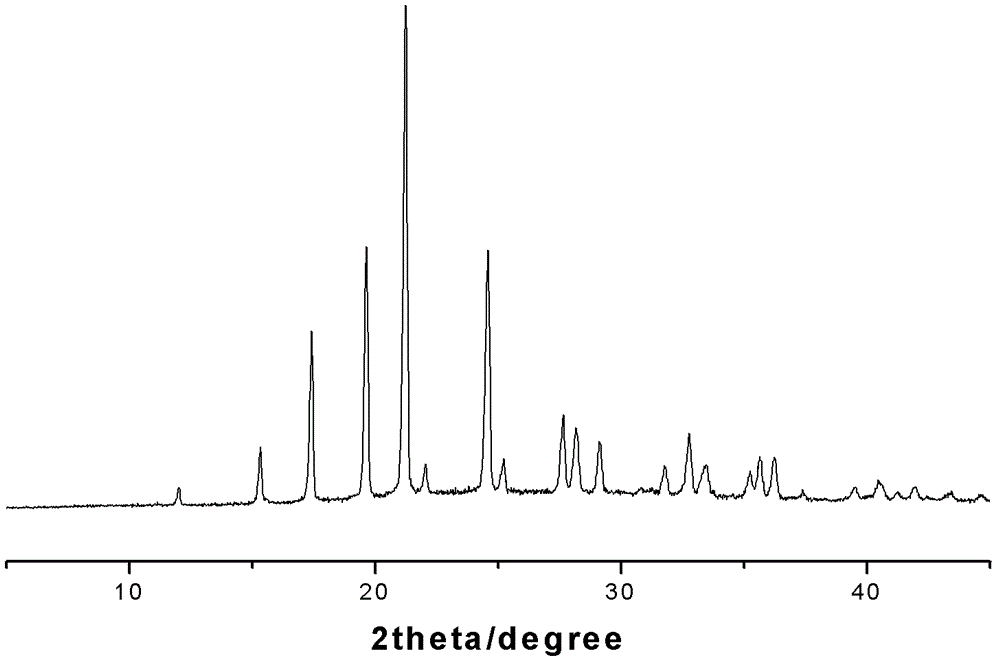
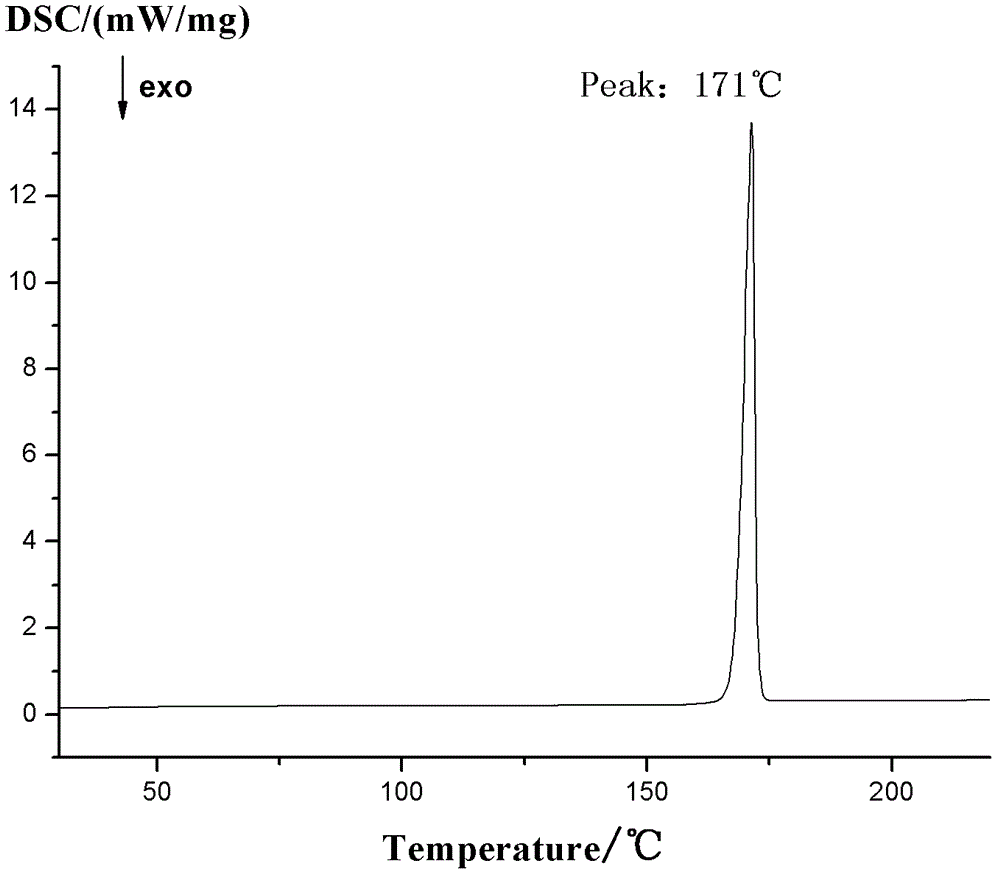
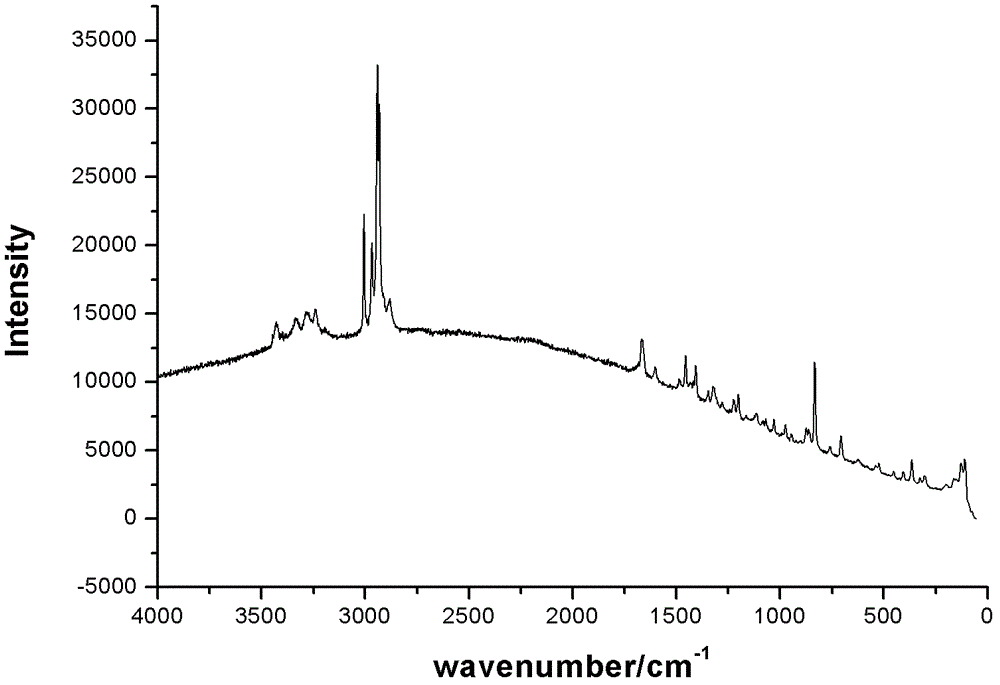
4-hydroxy-2-oxo-1-pyrrolidine acetamide racemate crystal I and preparation method thereof
A technology of pyrrolidine acetamide and racemate, which is applied in the field of 4-hydroxy-2-oxo-1-pyrrolidine acetamide medicine, and achieves the effects of being suitable for large-scale industrial production, high purity and remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Dissolve 50mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in anhydrous methanol, stir overnight, filter, put the solution in a desiccator, and evaporate the solvent to obtain colorless transparent crystals , yield 82%, purity 99.4%.
[0047] The crude product of 4-hydroxyl-2-oxo-1-pyrrolidineacetamide racemate of the present invention is synthesized according to the following steps, and its purity is 98.5%:
[0048] (a) Add 139.6g of glycine ethyl ester hydrochloride into 1100ml of anhydrous ether, cool to -6°C and blow in 27.2g of ammonia gas to free glycine ethyl ester hydrochloride into glycine ethyl ester, wherein glycine ethyl ester hydrochloride Salt: anhydrous ether: 1mol of ammonia: 1100ml: 1.6mol;
[0049] (b) Add 336ml of absolute ethanol, 67.2g of sodium bicarbonate, and 166.6g of 4-chloro-3-hydroxy-butyric acid ethyl ester dropwise to the above product. The adding time is 1.8 hours. React at 72°C for 22 hours;
[0050] (c) Filtration, ...
Embodiment 2
[0054] Dissolve 100mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in 95% ethanol, stir overnight, filter, put the solution in a desiccator, and evaporate the solvent to obtain colorless transparent crystals , yield 85%, purity 99.2%.
Embodiment 3
[0056] Dissolve 50 mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in isopropanol, stir overnight, filter, and place the solution in a desiccator to evaporate the solvent to obtain colorless transparent crystals , yield 87%, purity 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com