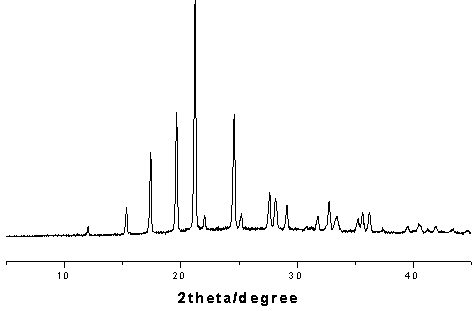
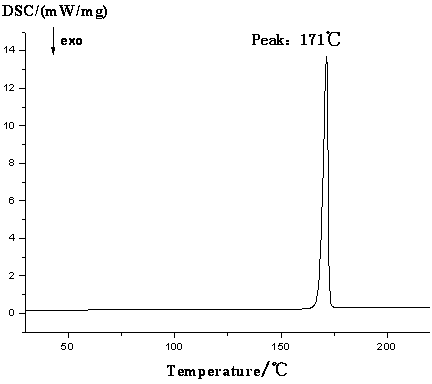
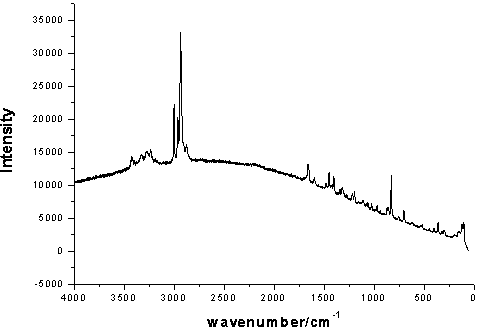
4-hydroxy-2-oxo-1-pyrrolidine acetamide racemate crystal i and preparation method thereof
A technology of pyrrolidine acetamide and racemate, applied in the field of 4-hydroxy-2-oxo-1-pyrrolidine acetamide racemate crystal form I and its preparation, achieving mild control conditions and a preparation method Simple, Powerful Results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Dissolve 50 mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in anhydrous methanol, stir overnight, filter, and place the solution in a desiccator to evaporate the solvent to obtain colorless transparent crystals. Yield 82%, purity 99.4%.
[0035] The 4-hydroxyl-2-oxo-1-pyrrolidineacetamide racemate crude product of the present invention is synthesized according to the following steps, and its purity is 98.5%:
[0036] (a) Add 139.6 g of ethyl glycine hydrochloride into 1100 ml of anhydrous ether, cool it to -6°C and blow in 27.2 g of ammonia gas to dissociate ethyl glycine hydrochloride into ethyl glycine, wherein ethyl glycine hydrochloride Salt: anhydrous ether: ammonia gas is 1mol: 1100ml: 1.6mol;
[0037] (b) Add absolute ethanol 336ml, sodium bicarbonate 67.2g, dropwise 166.6g of 4-chloro-3-hydroxyl-butyric acid ethyl ester in the above-mentioned product, and described dropping time is 1.8 hours, at pH8, temperature is React at 72°C for 22 hour...
Embodiment 2
[0042]Dissolve 100 mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in 95% ethanol, stir overnight, filter, and place the solution in a desiccator to evaporate the solvent to obtain colorless transparent crystals. Yield 85%, purity 99.2%.
Embodiment 3
[0044] Dissolve 50 mg of the crude racemate of 4-hydroxy-2-oxo-1-pyrrolidineacetamide in isopropanol, stir overnight, filter, and place the solution in a desiccator to evaporate the solvent to obtain colorless transparent crystals. Yield 87%, purity 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com